Polymerized cyclodextrin microparticles for sustained antibiotic delivery in lung infections
- PMID: 38380736
- PMCID: PMC11187681
- DOI: 10.1002/jbm.a.37680
Polymerized cyclodextrin microparticles for sustained antibiotic delivery in lung infections
Abstract
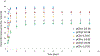
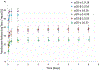
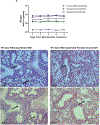
Pulmonary infections complicate chronic lung diseases requiring attention to both the pathophysiology and complexity associated with infection management. Patients with cystic fibrosis (CF) struggle with continuous bouts of pulmonary infections, contributing to lung destruction and eventual mortality. Additionally, CF patients struggle with airways that are highly viscous, with accumulated mucus creating optimal environments for bacteria colonization. The unique physiology and altered airway environment provide an ideal niche for bacteria to change their phenotype often becoming resistant to current treatments. Colonization with multiple pathogens at the same time further complicate treatment algorithms, requiring drug combinations that can challenge CF patient tolerance to treatment. The goal of this research initiative was to explore the utilization of a microparticle antibiotic delivery system, which could provide localized and sustained antibiotic dosing. The outcome of this work demonstrates the feasibility of providing efficient localized delivery of antibiotics to manage infection using both preclinical in vitro and in vivo CF infection models. The studies outlined in this manuscript demonstrate the proof-of-concept and unique capacity of polymerized cyclodextrin microparticles to provide site-directed management of pulmonary infections.
Keywords: Pseudomonas aeruginosa; cystic fibrosis; gentamicin; infection management; microparticle drug‐release; piperacillin; tazobactam; vancomycin.
© 2024 The Authors. Journal of Biomedical Materials Research Part A published by Wiley Periodicals LLC.
Figures









Similar articles
-
Antibiotic treatment for non-tuberculous mycobacteria lung infection in people with cystic fibrosis.Cochrane Database Syst Rev. 2025 Mar 27;3(3):CD016039. doi: 10.1002/14651858.CD016039. Cochrane Database Syst Rev. 2025. PMID: 40145528
-
Antibiotic strategies for eradicating Pseudomonas aeruginosa in people with cystic fibrosis.Cochrane Database Syst Rev. 2017 Apr 25;4(4):CD004197. doi: 10.1002/14651858.CD004197.pub5. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2023 Jun 2;6:CD004197. doi: 10.1002/14651858.CD004197.pub6. PMID: 28440853 Free PMC article. Updated.
-
Antibiotic strategies for eradicating Pseudomonas aeruginosa in people with cystic fibrosis.Cochrane Database Syst Rev. 2014 Nov 10;(11):CD004197. doi: 10.1002/14651858.CD004197.pub4. Cochrane Database Syst Rev. 2014. Update in: Cochrane Database Syst Rev. 2017 Apr 25;4:CD004197. doi: 10.1002/14651858.CD004197.pub5. PMID: 25383937 Updated.
-
Combination antimicrobial susceptibility testing for acute exacerbations in chronic infection of Pseudomonas aeruginosa in cystic fibrosis.Cochrane Database Syst Rev. 2015 Nov 2;(11):CD006961. doi: 10.1002/14651858.CD006961.pub3. Cochrane Database Syst Rev. 2015. Update in: Cochrane Database Syst Rev. 2017 Jun 19;6:CD006961. doi: 10.1002/14651858.CD006961.pub4. PMID: 26522473 Updated.
-
Macrolide antibiotics (including azithromycin) for cystic fibrosis.Cochrane Database Syst Rev. 2024 Feb 27;2(2):CD002203. doi: 10.1002/14651858.CD002203.pub5. Cochrane Database Syst Rev. 2024. PMID: 38411248 Free PMC article.
References
-
- Luyt C-E, Bouadma L, Morrris AC, Dhanani JA, Kollef M, Lipman J, Martin-Loeches I, Nseir S, Ranzani OT, Roquilly A, SChmidt M, Torres A, Timsit J-F. Pulmonary Infections Complicating ARDS. Intensive Cre Med. 2020. 2020. Dec;46(12):2168–2183. doi: 10.1007/s00134-020-06292-z. Epub 2020 Nov 11. - DOI - PMC - PubMed
-
- Wi YM, Choi JY, Lee J-Y, Kang C-I, Chung DR, Peck KR, Song J-H, Ko KS. The Antimicrobial Effects of -lactams on Imipenem-Resistant Ceftazidime-Susceptible Pseudomonas aeruginosa. 2017. Antimicrob Agents Chemother 2017. May 24;61(6): e00054–17. doi: 10.1128/AAC.00054-17. Print 2017 Jun. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

