Maturation of glutamatergic transmission onto dorsal raphe serotonergic neurons
- PMID: 38380827
- PMCID: PMC11305679
- DOI: 10.1152/jn.00037.2023
Maturation of glutamatergic transmission onto dorsal raphe serotonergic neurons
Abstract
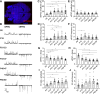
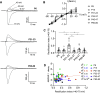
Serotonergic neurons in the dorsal raphe nucleus (DRN) play important roles early in postnatal development in the maturation and modulation of higher-order emotional, sensory, and cognitive circuitry. The pivotal functions of these cells in brain development make them a critical substrate by which early experience can be wired into the brain. In this study, we investigated the maturation of synapses onto dorsal raphe serotonergic neurons in typically developing male and female mice using whole cell patch-clamp recordings in ex vivo brain slices. We show that while inhibition of these neurons is relatively stable across development, glutamatergic synapses greatly increase in strength between postnatal day 6 (P6) and P21-23. In contrast to forebrain regions, where the components making up glutamatergic synapses are dynamic across early life, we find that DRN excitatory synapses maintain a very high ratio of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) to N-methyl-d-aspartate (NMDA) receptors and a rectifying component of the AMPA response until adulthood. Overall, these findings reveal that the development of serotonergic neurons is marked by a significant refinement of glutamatergic synapses during the first three postnatal weeks. This suggests this time is a sensitive period of heightened plasticity for the integration of information from upstream brain areas. Genetic and environmental insults during this period could lead to alterations in serotonergic output, impacting both the development of forebrain circuits and lifelong neuromodulatory actions.NEW & NOTEWORTHY Serotonergic neurons are regulators of both the development of and ongoing activity in neuronal circuits controlling affective, cognitive, and sensory processing. Here, we characterize the maturation of extrinsic synaptic inputs onto these cells, showing that the first three postnatal weeks are a period of synaptic refinement and a potential window for experience-dependent plasticity in response to both enrichment and adversity.
Keywords: development; dorsal raphe; excitatory synapse; glutamate; serotonin.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures



Update of
-
Maturation of glutamatergic transmission onto dorsal raphe serotonergic neurons.bioRxiv [Preprint]. 2023 Jan 19:2023.01.19.524776. doi: 10.1101/2023.01.19.524776. bioRxiv. 2023. Update in: J Neurophysiol. 2024 Apr 1;131(4):626-637. doi: 10.1152/jn.00037.2023. PMID: 36711665 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

