Presenilin-1 mutation position influences amyloidosis, small vessel disease, and dementia with disease stage
- PMID: 38380882
- PMCID: PMC11032566
- DOI: 10.1002/alz.13729
Presenilin-1 mutation position influences amyloidosis, small vessel disease, and dementia with disease stage
Abstract
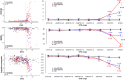
Introduction: Amyloidosis, including cerebral amyloid angiopathy, and markers of small vessel disease (SVD) vary across dominantly inherited Alzheimer's disease (DIAD) presenilin-1 (PSEN1) mutation carriers. We investigated how mutation position relative to codon 200 (pre-/postcodon 200) influences these pathologic features and dementia at different stages.
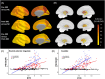
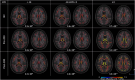
Methods: Individuals from families with known PSEN1 mutations (n = 393) underwent neuroimaging and clinical assessments. We cross-sectionally evaluated regional Pittsburgh compound B-positron emission tomography uptake, magnetic resonance imaging markers of SVD (diffusion tensor imaging-based white matter injury, white matter hyperintensity volumes, and microhemorrhages), and cognition.
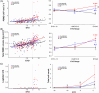
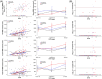
Results: Postcodon 200 carriers had lower amyloid burden in all regions but worse markers of SVD and worse Clinical Dementia Rating® scores compared to precodon 200 carriers as a function of estimated years to symptom onset. Markers of SVD partially mediated the mutation position effects on clinical measures.
Discussion: We demonstrated the genotypic variability behind spatiotemporal amyloidosis, SVD, and clinical presentation in DIAD, which may inform patient prognosis and clinical trials.
Highlights: Mutation position influences Aβ burden, SVD, and dementia. PSEN1 pre-200 group had stronger associations between Aβ burden and disease stage. PSEN1 post-200 group had stronger associations between SVD markers and disease stage. PSEN1 post-200 group had worse dementia score than pre-200 in late disease stage. Diffusion tensor imaging-based SVD markers mediated mutation position effects on dementia in the late stage.
Keywords: PSEN1; PiB‐PET; autosomal dominant Alzheimer's disease (ADAD); cerebral amyloid angiopathy (CAA); codon 200; dominantly inherited Alzheimer's disease (DIAD); microbleeds; microhemorrhages; peak width of skeletonized mean diffusivity (PSMD); presenilin‐1; small vessel disease (SVD); white matter hyperintensity (WMH).
© 2024 The Authors. Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
NJM reports receiving a travel fellowship from the Alzheimer's Association to present related work at the Alzheimer Imaging Consortium preconference and the Alzheimer's Association International Conference. CC has received research support from GSK and Eisai Co., Ltd., and acknowledges travel support from Somalogics and consulting fees from Circular Genomics and Alector. CC is a member of the advisory board of Vivid Genetics and Circular Genomics and owns stock in those companies. DMC reports travel support from the Alzheimer's Association. GSD reports consulting fees from Parabon Nanolabs, honoraria from PeerView Media, Continuing Education Inc, Eli Lilly and Company, expert testimony fees from Barrow and from DynaMed, material support from Horizon Therapeutics and Avid Radiopharmaceuticals, and owing stock in ANI Pharmaceuticals and Parabon Nanolabs. NCF has provided consultancy or served on advisory or data safety and monitoring boards for F. Hoffmann‐La Roche, Ltd. and Eli Lilly and Company, Ionis, Biogen, and Siemens. WEK is a co‐inventor on a patent portfolio related to PiB PET technology owned by the University of Pittsburgh. GE Healthcare holds a license agreement with the University of Pittsburgh based on the technology described in this manuscript. Dr. Klunk is a co‐inventor of PiB and, as such, has a financial interest in this license agreement. GE Healthcare provided no grant support for this study and had no role in the design or interpretation of results or preparation of this manuscript. WEK has served on the data safety monitoring board for Biogen and owns stock in Cognoptix. JL reports consulting fees and/or honoraria from Eisai Co., Ltd., Biogen, Bayer Vital, TEVA, F. Hoffmann‐La Roche, Ltd., and Zambon, participation on an advisory board for Axon Neuroscience. JMR reports research support from Avid Radiopharmaceuticals. AJS has served on advisory committees for Eisai Co., Ltd., and Siemens Medical Solutions USA, Inc., and has received material from Avid Radiopharmaceuticals, a subsidiary of Eli Lilly and Company. PRS is the company director for Neuroscience Research Australian Foundation (NeuRA), the Health‐Science Alliance, Schizophrenia Research Institute, Australian Association of Medical Research Institutes, Australia Dementia Network Ltd., Standing Pty Ltd., and Australasian Neuroscience Society and acknowledges receiving consulting fees from NeuRA. IY reports consulting fees from ABX‐CRO and Blue Earth Diagnostics, honoraria from Piramal, and travel supports from the Society of Nuclear Medicine and Molecular Imaging (SNMMI) and the European Association of Nuclear Medicine, for both of which he has served on committee. AMB reports consulting fees from Cognition Therapeutics and Regeneron, honoraria from Yale University, Celdara Medical, International Neuropsychological Society, Pennington Biomedical Research Center, International Society for Neurovascular Disease, American College of Neuropsychopharmacology, American Neuropathologists, travel support from the International Neuropsychological Society. AMB has served on an advisory board for the Albert Einstein College of Medicine and CogState and is an editor for
Figures

References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
- R01 AG069453/AG/NIA NIH HHS/United States
- SG-20-690363-DIAN/ALZ/Alzheimer's Association/United States
- RF1 AG073424/AG/NIA NIH HHS/United States
- K01 AG084816/AG/NIA NIH HHS/United States
- P30 AG066530/AG/NIA NIH HHS/United States
- R03AG072375/AG/NIA NIH HHS/United States
- R01AG074909/AG/NIA NIH HHS/United States
- P30AG072980/AG/NIA NIH HHS/United States
- AARFD-20-681815/ALZ/Alzheimer's Association/United States
- U19 AG032438/AG/NIA NIH HHS/United States
- R03 AG072375/AG/NIA NIH HHS/United States
- 1K01AG080123/AG/NIA NIH HHS/United States
- U19AG032438/AG/NIA NIH HHS/United States
- P30 AG072980/AG/NIA NIH HHS/United States
- T32 AG058518/AG/NIA NIH HHS/United States
- AARF-21-722077/ALZ/Alzheimer's Association/United States
- K01 AG080123/AG/NIA NIH HHS/United States
- T32 AG078117/AG/NIA NIH HHS/United States
- R01 AG074909/AG/NIA NIH HHS/United States
- T32AG078117-01/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

