Influence of RNA circularity on Target RNA-Directed MicroRNA Degradation
- PMID: 38381063
- PMCID: PMC11014252
- DOI: 10.1093/nar/gkae094
Influence of RNA circularity on Target RNA-Directed MicroRNA Degradation
Abstract
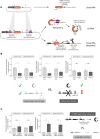
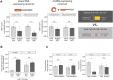
A subset of circular RNAs (circRNAs) and linear RNAs have been proposed to 'sponge' or block microRNA activity. Additionally, certain RNAs induce microRNA destruction through the process of Target RNA-Directed MicroRNA Degradation (TDMD), but whether both linear and circular transcripts are equivalent in driving TDMD is unknown. Here, we studied whether circular/linear topology of endogenous and artificial RNA targets affects TDMD. Consistent with previous knowledge that Cdr1as (ciRS-7) circular RNA protects miR-7 from Cyrano-mediated TDMD, we demonstrate that depletion of Cdr1as reduces miR-7 abundance. In contrast, overexpression of an artificial linear version of Cdr1as drives miR-7 degradation. Using plasmids that express a circRNA with minimal co-expressed cognate linear RNA, we show differential effects on TDMD that cannot be attributed to the nucleotide sequence, as the TDMD properties of a sequence often differ when in a circular versus linear form. By analysing RNA sequencing data of a neuron differentiation system, we further detect potential effects of circRNAs on microRNA stability. Our results support the view that RNA circularity influences TDMD, either enhancing or inhibiting it on specific microRNAs.
© The Author(s) 2024. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures





References
-
- Hansen T.B., Jensen T.I., Clausen B.H., Bramsen J.B., Finsen B., Damgaard C.K., Kjems J.. Natural RNA circles function as efficient microRNA sponges. Nature. 2013; 495:384–388. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

