Marginal zone B cells produce 'natural' atheroprotective IgM antibodies in a T cell-dependent manner
- PMID: 38381113
- PMCID: PMC10939463
- DOI: 10.1093/cvr/cvae027
Marginal zone B cells produce 'natural' atheroprotective IgM antibodies in a T cell-dependent manner
Abstract
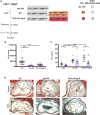
Aims: The adaptive immune response plays an important role in atherosclerosis. In response to a high-fat/high-cholesterol (HF/HC) diet, marginal zone B (MZB) cells activate an atheroprotective programme by regulating the differentiation and accumulation of 'poorly differentiated' T follicular helper (Tfh) cells. On the other hand, Tfh cells activate the germinal centre response, which promotes atherosclerosis through the production of class-switched high-affinity antibodies. We therefore investigated the direct role of Tfh cells and the role of IL18 in Tfh differentiation in atherosclerosis.
Methods and results: We generated atherosclerotic mouse models with selective genetic deletion of Tfh cells, MZB cells, or IL18 signalling in Tfh cells. Surprisingly, mice lacking Tfh cells had increased atherosclerosis. Lack of Tfh not only reduced class-switched IgG antibodies against oxidation-specific epitopes (OSEs) but also reduced atheroprotective natural IgM-type anti-phosphorylcholine (PC) antibodies, despite no alteration of natural B1 cells. Moreover, the absence of Tfh cells was associated with an accumulation of MZB cells with substantially reduced ability to secrete antibodies. In the same manner, MZB cell deficiency in Ldlr-/- mice was associated with a significant decrease in atheroprotective IgM antibodies, including natural anti-PC IgM antibodies. In humans, we found a positive correlation between circulating MZB-like cells and anti-OSE IgM antibodies. Finally, we identified an important role for IL18 signalling in HF/HC diet-induced Tfh.
Conclusion: Our findings reveal a previously unsuspected role of MZB cells in regulating atheroprotective 'natural' IgM antibody production in a Tfh-dependent manner, which could have important pathophysiological and therapeutic implications.
Keywords: Antibodies; Atherosclerosis; B cells; Interleukin-18; T cells.
© The Author(s) 2024. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest: none declared.
Figures





References
-
- Centa M, Jin H, Hofste L, Hellberg S, Busch A, Baumgartner R, Verzaal NJ, Lind Enoksson S, Perisic Matic L, Boddul SV, Atzler D, Li DY, Sun C, Hansson GK, Ketelhuth DFJ, Hedin U, Wermeling F, Lutgens E, Binder CJ, Maegdesfessel L, Malin SG. Germinal center-derived antibodies promote atherosclerosis plaque size and stability. Circulation 2019;139:2466–2482. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

