Targeting Lipoprotein(a): Can RNA Therapeutics Provide the Next Step in the Prevention of Cardiovascular Disease?
- PMID: 38381282
- PMCID: PMC10899152
- DOI: 10.1007/s40119-024-00353-w
Targeting Lipoprotein(a): Can RNA Therapeutics Provide the Next Step in the Prevention of Cardiovascular Disease?
Abstract
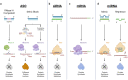
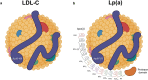
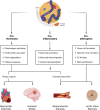
Numerous genetic and epidemiologic studies have demonstrated an association between elevated levels of lipoprotein(a) (Lp[a]) and cardiovascular disease. As a result, lowering Lp(a) levels is widely recognized as a promising strategy for reducing the risk of new-onset coronary heart disease, stroke, and heart failure. Lp(a) consists of a low-density lipoprotein-like particle with covalently linked apolipoprotein A (apo[a]) and apolipoprotein B-100, which explains its pro-thrombotic, pro-inflammatory, and pro-atherogenic properties. Lp(a) serum concentrations are genetically determined by the apo(a) isoform, with shorter isoforms having a higher rate of particle synthesis. To date, there are no approved pharmacological therapies that effectively reduce Lp(a) levels. Promising treatment approaches targeting apo(a) expression include RNA-based drugs such as pelacarsen, olpasiran, SLN360, and lepodisiran, which are currently in clinical trials. In this comprehensive review, we provide a detailed overview of RNA-based therapeutic approaches and discuss the recent advances and challenges of RNA therapeutics specifically designed to reduce Lp(a) levels and thus the risk of cardiovascular disease.
Keywords: Cardiovascular disease; Elevated Lp(a) levels; Lepodisiran (LY3819469); Lipoprotein(a); Olpasiran; Pelacarsen; RNA therapeutics; SLN360; Therapy.
© 2024. The Author(s).
Conflict of interest statement
Henriette Thau, Sebastian Neuber, Maximilian Y. Emmert and Timo Z. Nazari-Shafti declare no conflict of interest and no commercial or financial relationships.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

