Spontaneous, persistent, T cell-dependent IFN-γ release in patients who progress to Long Covid
- PMID: 38381822
- PMCID: PMC10881041
- DOI: 10.1126/sciadv.adi9379
Spontaneous, persistent, T cell-dependent IFN-γ release in patients who progress to Long Covid
Abstract
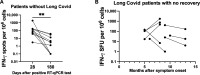
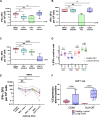
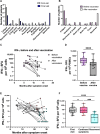
After acute infection with severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), a proportion of patients experience persistent symptoms beyond 12 weeks, termed Long Covid. Understanding the mechanisms that cause this debilitating disease and identifying biomarkers for diagnostic, therapeutic, and monitoring purposes are urgently required. We detected persistently high levels of interferon-γ (IFN-γ) from peripheral blood mononuclear cells of patients with Long Covid using highly sensitive FluoroSpot assays. This IFN-γ release was seen in the absence of ex vivo peptide stimulation and remains persistently elevated in patients with Long Covid, unlike the resolution seen in patients recovering from acute SARS-CoV-2 infection. The IFN-γ release was CD8+ T cell-mediated and dependent on antigen presentation by CD14+ cells. Longitudinal follow-up of our study cohort showed that symptom improvement and resolution correlated with a decrease in IFN-γ production to baseline levels. Our study highlights a potential mechanism underlying Long Covid, enabling the search for biomarkers and therapeutics in patients with Long Covid.
Figures


References
-
- Scherlinger M., Felten R., Gallais F., Nazon C., Chatelus E., Pijnenburg L., Mengin A., Gras A., Vidailhet P., Arnould-Michel R., Bibi-Triki S., Carapito R., Trouillet-Assant S., Perret M., Belot A., Bahram S., Arnaud L., Gottenberg J. E., Fafi-Kremer S., Sibilia J., Refining “long-COVID” by a prospective multimodal evaluation of patients with long-term symptoms attributed to SARS-CoV-2 infection. Infect. Dis. Ther. 10, 1747–1763 (2021). - PMC - PubMed
-
- Huang C., Huang L., Wang Y., Li X., Ren L., Gu X., Kang L., Guo L., Liu M., Zhou X., Luo J., Huang Z., Tu S., Zhao Y., Chen L., Xu D., Li Y., Li C., Peng L., Li Y., Xie W., Cui D., Shang L., Fan G., Xu J., Wang G., Wang Y., Zhong J., Wang C., Wang J., Zhang D., Cao B., 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet 397, 220–232 (2021). - PMC - PubMed
-
- Gupta A., Madhavan M. V., Sehgal K., Nair N., Mahajan S., Sehrawat T. S., Bikdeli B., Ahluwalia N., Ausiello J. C., Wan E. Y., Freedberg D. E., Kirtane A. J., Parikh S. A., Maurer M. S., Nordvig A. S., Accili D., Bathon J. M., Mohan S., Bauer K. A., Leon M. B., Krumholz H. M., Uriel N., Mehra M. R., Elkind M. S. V., Stone G. W., Schwartz A., Ho D. D., Bilezikian J. P., Landry D. W., Extrapulmonary manifestations of COVID-19. Nat. Med. 26, 1017–1032 (2020). - PMC - PubMed
-
- Thompson E. J., Williams D. M., Walker A. J., Mitchell R. E., Niedzwiedz C. L., Yang T. C., Huggins C. F., Kwong A. S. F., Silverwood R. J., di Gessa G., Bowyer R. C. E., Northstone K., Hou B., Green M. J., Dodgeon B., Doores K. J., Duncan E. L., Williams F. M. K., OpenSAFELY Collaborative, Walker A. J., MacKenna B., Inglesby P., Rentsch C. T., Curtis H. J., Morton C. E., Morley J., Mehrkar A., Bacon S., Hickman G., Bates C., Croker R., Evans D., Ward T., Cockburn J., Davy S., Bhaskaran K., Schultze A., Williamson E. J., Hulme W. J., McDonald H. I., Tomlinson L., Mathur R., Eggo R. M., Wing K., Wong A. Y. S., Forbes H., Tazare J., Parry J., Hester F., Harper S., Douglas I. J., Evans S. J. W., Smeeth L., Goldacre B., Steptoe A., Porteous D. J., McEachan R. R. C., Tomlinson L., Goldacre B., Patalay P., Ploubidis G. B., Katikireddi S. V., Tilling K., Rentsch C. T., Timpson N. J., Chaturvedi N., Steves C. J., Long COVID burden and risk factors in 10 UK longitudinal studies and electronic health records. Nat. Commun. 13, 3528 (2022). - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

