Lipid nanoparticles and siRNA targeting plasminogen provide lasting inhibition of fibrinolysis in mouse and dog models of hemophilia A
- PMID: 38381848
- PMCID: PMC11293256
- DOI: 10.1126/scitranslmed.adh0027
Lipid nanoparticles and siRNA targeting plasminogen provide lasting inhibition of fibrinolysis in mouse and dog models of hemophilia A
Abstract
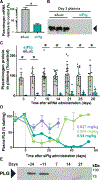
Antifibrinolytic drugs are used extensively for on-demand treatment of severe acute bleeding. Controlling fibrinolysis may also be an effective strategy to prevent or lessen chronic recurring bleeding in bleeding disorders such as hemophilia A (HA), but current antifibrinolytics have unfavorable pharmacokinetic profiles. Here, we developed a long-lasting antifibrinolytic using small interfering RNA (siRNA) targeting plasminogen packaged in clinically used lipid nanoparticles (LNPs) and tested it to determine whether reducing plasmin activity in animal models of HA could decrease bleeding frequency and severity. Treatment with the siRNA-carrying LNPs reduced circulating plasminogen and suppressed fibrinolysis in wild-type and HA mice and dogs. In HA mice, hemostatic efficacy depended on the injury model; plasminogen knockdown improved hemostasis after a saphenous vein injury but not tail vein transection injury, suggesting that saphenous vein injury is a murine bleeding model sensitive to the contribution of fibrinolysis. In dogs with HA, LNPs carrying siRNA targeting plasminogen were as effective at stabilizing clots as tranexamic acid, a clinical antifibrinolytic, and in a pilot study of two dogs with HA, the incidence of spontaneous or excess bleeding was reduced during 4 months of prolonged knockdown. Collectively, these data demonstrate that long-acting antifibrinolytic therapy can be achieved and that it provides hemostatic benefit in animal models of HA.
Conflict of interest statement
Figures



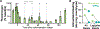
References
-
- Nesheim M, Thrombin and fibrinolysis. Chest 124 (3 Suppl), 33S–39S (2003). - PubMed
-
- Cesarman-Maus G, Hajjar KA, Molecular mechanisms of fibrinolysis. Br. J. Haematol. 129, 307–321 (2005). - PubMed
-
- He S, Blombäck M, Jacobsson Ekman G, Hedner U, The role of recombinant factor VIIa (FVIIa) in fibrin structure in the absence of FVIII/FIX. J. Thromb. Haemost. 1, 1215–1219 (2003). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

