Pathogenic CANVAS (AAGGG)n repeats stall DNA replication due to the formation of alternative DNA structures
- PMID: 38381906
- PMCID: PMC11077069
- DOI: 10.1093/nar/gkae124
Pathogenic CANVAS (AAGGG)n repeats stall DNA replication due to the formation of alternative DNA structures
Abstract
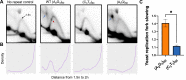
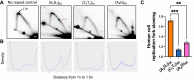
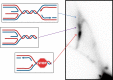
CANVAS is a recently characterized repeat expansion disease, most commonly caused by homozygous expansions of an intronic (A2G3)n repeat in the RFC1 gene. There are a multitude of repeat motifs found in the human population at this locus, some of which are pathogenic and others benign. In this study, we conducted structure-functional analyses of the pathogenic (A2G3)n and nonpathogenic (A4G)n repeats. We found that the pathogenic, but not the nonpathogenic, repeat presents a potent, orientation-dependent impediment to DNA polymerization in vitro. The pattern of the polymerization blockage is consistent with triplex or quadruplex formation in the presence of magnesium or potassium ions, respectively. Chemical probing of both repeats in vitro reveals triplex H-DNA formation by only the pathogenic repeat. Consistently, bioinformatic analysis of S1-END-seq data from human cell lines shows preferential H-DNA formation genome-wide by (A2G3)n motifs over (A4G)n motifs. Finally, the pathogenic, but not the nonpathogenic, repeat stalls replication fork progression in yeast and human cells. We hypothesize that the CANVAS-causing (A2G3)n repeat represents a challenge to genome stability by folding into alternative DNA structures that stall DNA replication.
© The Author(s) 2024. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures




Update of
-
Pathogenic CANVAS (AAGGG)n repeats stall DNA replication due to the formation of alternative DNA structures.bioRxiv [Preprint]. 2023 Jul 26:2023.07.25.550509. doi: 10.1101/2023.07.25.550509. bioRxiv. 2023. Update in: Nucleic Acids Res. 2024 May 8;52(8):4361-4374. doi: 10.1093/nar/gkae124. PMID: 37546920 Free PMC article. Updated. Preprint.
References
-
- Rafehi H., Szmulewicz D.J., Bennett M.F., Sobreira N.L.M., Pope K., Smith K.R., Gillies G., Diakumis P., Dolzhenko E., Eberle M.A.et al. .. Bioinformatics-based identification of expanded repeats: a non-reference intronic pentamer expansion in RFC1 causes CANVAS. Am. J. Hum. Genet. 2019; 105:151–165. - PMC - PubMed
-
- Arteche-López A., Avila-Fernandez A., Damian A., Soengas-Gonda E., de la Fuente R.P., Gómez P.R., Merlo J.G., Burgos L.H., Fernández C.C., Rosales J.M.L.et al. .. New Cerebellar Ataxia, Neuropathy, Vestibular Areflexia Syndrome cases are caused by the presence of a nonsense variant in compound heterozygosity with the pathogenic repeat expansion in the RFC1 gene. Clin. Genet. 2023; 103:236–241. - PubMed
-
- Cortese A., Curro’ R., Vegezzi E., Yau W.Y., Houlden H., Reilly M.M. Cerebellar ataxia, neuropathy and vestibular areflexia syndrome (CANVAS): genetic and clinical aspects. Pract. Neurol. 2022; 22:14–18. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

