US Food and Drug Administration Approval Summary: Elacestrant for Estrogen Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative, ESR1-Mutated Advanced or Metastatic Breast Cancer
- PMID: 38381994
- PMCID: PMC11003513
- DOI: 10.1200/JCO.23.02112
US Food and Drug Administration Approval Summary: Elacestrant for Estrogen Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative, ESR1-Mutated Advanced or Metastatic Breast Cancer
Abstract
Purpose: The US Food and Drug Administration (FDA) approved elacestrant for the treatment of postmenopausal women or adult men with estrogen receptor-positive (ER+), human epidermal growth factor receptor 2-negative (HER2-), estrogen receptor 1 (ESR1)-mutated advanced or metastatic breast cancer with disease progression after at least one line of endocrine therapy (ET).
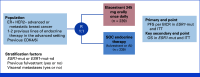
Patients and methods: Approval was based on EMERALD (Study RAD1901-308), a randomized, open-label, active-controlled, multicenter trial in 478 patients with ER+, HER2- advanced or metastatic breast cancer, including 228 patients with ESR1 mutations. Patients were randomly assigned (1:1) to receive either elacestrant 345 mg orally once daily (n = 239) or investigator's choice of ET (n = 239).
Results: In the ESR1-mut subgroup, EMERALD demonstrated a statistically significant improvement in progression-free survival (PFS) by blinded independent central review assessment (n = 228; hazard ratio [HR], 0.55 [95% CI, 0.39 to 0.77]; P value = .0005). Although the overall survival (OS) end point was not met, there was no trend toward a potential OS detriment (HR, 0.90 [95% CI, 0.63 to 1.30]) in the ESR1-mut subgroup. PFS also reached statistical significance in the intention-to-treat population (ITT, N = 478; HR, 0.70 [95% CI, 0.55 to 0.88]; P value = .0018). However, improvement in PFS in the ITT population was primarily attributed to results from patients in the ESR1-mut subgroup. More patients who received elacestrant experienced nausea, vomiting, and dyslipidemia.
Conclusion: The approval of elacestrant in ER+, HER2- advanced or metastatic breast cancer was restricted to patients with ESR1 mutations. Benefit-risk assessment in the ESR1-mut subgroup was favorable on the basis of a statistically significant improvement in PFS in the context of an acceptable safety profile including no evidence of a potential detriment in OS. By contrast, the benefit-risk assessment in patients without ESR1 mutations was not favorable. Elacestrant is the first oral estrogen receptor antagonist to receive FDA approval for patients with ESR1 mutations.
Trial registration: ClinicalTrials.gov NCT03778931.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
No other potential conflicts of interest were reported.
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

