Not so hidden anymore: Advances and challenges in understanding root growth under water deficits
- PMID: 38382086
- PMCID: PMC11062450
- DOI: 10.1093/plcell/koae055
Not so hidden anymore: Advances and challenges in understanding root growth under water deficits
Erratum in
-
Correction to: Not so hidden anymore: Advances and challenges in understanding root growth under water deficits.Plant Cell. 2024 Jul 31;36(8):2927-2928. doi: 10.1093/plcell/koae150. Plant Cell. 2024. PMID: 38837717 Free PMC article. No abstract available.
Abstract
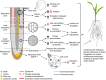
Limited water availability is a major environmental factor constraining plant development and crop yields. One of the prominent adaptations of plants to water deficits is the maintenance of root growth that enables sustained access to soil water. Despite early recognition of the adaptive significance of root growth maintenance under water deficits, progress in understanding has been hampered by the inherent complexity of root systems and their interactions with the soil environment. We highlight selected milestones in the understanding of root growth responses to water deficits, with emphasis on founding studies that have shaped current knowledge and set the stage for further investigation. We revisit the concept of integrated biophysical and metabolic regulation of plant growth and use this framework to review central growth-regulatory processes occurring within root growth zones under water stress at subcellular to organ scales. Key topics include the primary processes of modifications of cell wall-yielding properties and osmotic adjustment, as well as regulatory roles of abscisic acid and its interactions with other hormones. We include consideration of long-recognized responses for which detailed mechanistic understanding has been elusive until recently, for example hydrotropism, and identify gaps in knowledge, ongoing challenges, and opportunities for future research.
© The Author(s) 2024. Published by Oxford University Press on behalf of American Society of Plant Biologists. All rights reserved. For commercial re-use, please contact reprints@oup.com for reprints and translation rights for reprints. All other permissions can be obtained through our RightsLink service via the Permissions link on the article page on our site—for further information please contact journals.permissions@oup.com.
Conflict of interest statement
Conflict of interest statement. None declared.
Figures






Similar articles
-
To grow or not to grow: the enigma of plant root growth dynamism.Plant Mol Biol. 2025 Jul 30;115(4):93. doi: 10.1007/s11103-025-01631-4. Plant Mol Biol. 2025. PMID: 40736880 Review.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
Clinical symptoms, signs and tests for identification of impending and current water-loss dehydration in older people.Cochrane Database Syst Rev. 2015 Apr 30;2015(4):CD009647. doi: 10.1002/14651858.CD009647.pub2. Cochrane Database Syst Rev. 2015. PMID: 25924806 Free PMC article.
-
Psychological interventions for adults who have sexually offended or are at risk of offending.Cochrane Database Syst Rev. 2012 Dec 12;12(12):CD007507. doi: 10.1002/14651858.CD007507.pub2. Cochrane Database Syst Rev. 2012. PMID: 23235646 Free PMC article.
-
Autotoxin Rg1 Induces Degradation of Root Cell Walls and Aggravates Root Rot by Modifying the Rhizospheric Microbiome.Microbiol Spectr. 2021 Dec 22;9(3):e0167921. doi: 10.1128/spectrum.01679-21. Epub 2021 Dec 15. Microbiol Spectr. 2021. PMID: 34908454 Free PMC article.
Cited by
-
Resilience of Maize to Environmental Stress: Insights into Drought and Heat Tolerance.Int J Mol Sci. 2025 May 30;26(11):5274. doi: 10.3390/ijms26115274. Int J Mol Sci. 2025. PMID: 40508082 Free PMC article. Review.
-
Translational insights into abiotic interactions: From Arabidopsis to crop plants.Plant Cell. 2025 Jul 1;37(7):koaf140. doi: 10.1093/plcell/koaf140. Plant Cell. 2025. PMID: 40579370 Free PMC article. Review.
-
Understanding and optimizing plant growth in water-limited environments: 'growth versus defence' or 'growth versus risk mitigation'?Philos Trans R Soc Lond B Biol Sci. 2025 May 29;380(1927):20240232. doi: 10.1098/rstb.2024.0232. Epub 2025 May 29. Philos Trans R Soc Lond B Biol Sci. 2025. PMID: 40439302
-
Celebrating the American Society of Plant Biologists centennial anniversary: A compendium of review articles in plant biology.Plant Cell. 2024 May 1;36(5):1183-1185. doi: 10.1093/plcell/koae076. Plant Cell. 2024. PMID: 38466716 Free PMC article. No abstract available.
-
Celebrating the American Society of Plant Biologists centennial anniversary: A compendium of review articles in plant biology.Plant Physiol. 2024 Apr 30;195(1):1-3. doi: 10.1093/plphys/kiae141. Plant Physiol. 2024. PMID: 38547371 Free PMC article. No abstract available.
References
-
- Ahmed MA, Zarebanadkouki M, Kaestner A, Carminati A. Measurements of water uptake of maize roots: the key function of lateral roots. Plant Soil. 2016:398(1-2):59–77. 10.1007/s11104-015-2639-6 - DOI
-
- Augé RM. Water relations, drought and vesicular-arbuscular mycorrhizal symbiosis. Mycorrhiza. 2001:11(1):3–42. 10.1007/s005720100097 - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

