Cholesterol and Lipid Rafts in the Biogenesis of Amyloid-β Protein and Alzheimer's Disease
- PMID: 38382114
- PMCID: PMC11575466
- DOI: 10.1146/annurev-biophys-062823-023436
Cholesterol and Lipid Rafts in the Biogenesis of Amyloid-β Protein and Alzheimer's Disease
Abstract
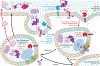
Cholesterol has been conjectured to be a modulator of the amyloid cascade, the mechanism that produces the amyloid-β (Aβ) peptides implicated in the onset of Alzheimer's disease. We propose that cholesterol impacts the genesis of Aβ not through direct interaction with proteins in the bilayer, but indirectly by inducing the liquid-ordered phase and accompanying liquid-liquid phase separations, which partition proteins in the amyloid cascade to different lipid domains and ultimately to different endocytotic pathways. We explore the full process of Aβ genesis in the context of liquid-ordered phases induced by cholesterol, including protein partitioning into lipid domains, mechanisms of endocytosis experienced by lipid domains and secretases, and pH-controlled activation of amyloid precursor protein secretases in specific endocytotic environments. Outstanding questions on the essential role of cholesterol in the amyloid cascade are identified for future studies.
Keywords: amyloid precursor protein; cellular compartment; cholesterol; lipid bilayer; lipid phase; molecular dynamics.
Figures





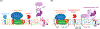
Similar articles
-
CSF tau and the CSF tau/ABeta ratio for the diagnosis of Alzheimer's disease dementia and other dementias in people with mild cognitive impairment (MCI).Cochrane Database Syst Rev. 2017 Mar 22;3(3):CD010803. doi: 10.1002/14651858.CD010803.pub2. Cochrane Database Syst Rev. 2017. PMID: 28328043 Free PMC article.
-
Co-Aggregation of Syndecan-3 with β-Amyloid Aggravates Neuroinflammation and Cognitive Impairment in 5×FAD Mice.Int J Mol Sci. 2025 Jun 8;26(12):5502. doi: 10.3390/ijms26125502. Int J Mol Sci. 2025. PMID: 40564963 Free PMC article.
-
Altered amyloid-β structure markedly reduces gliosis in the brain of mice harboring the Uppsala APP deletion.Acta Neuropathol Commun. 2024 Feb 5;12(1):22. doi: 10.1186/s40478-024-01734-x. Acta Neuropathol Commun. 2024. PMID: 38317196 Free PMC article.
-
Acute targeting of N-terminal tau protein has long-lasting beneficial effects in Tg2576 APP/Aβ mouse model by reducing cognitive impairment, cerebral Aβ-amyloidosis, synaptic remodeling and microgliosis later in life.Acta Neuropathol Commun. 2025 May 29;13(1):121. doi: 10.1186/s40478-025-02022-y. Acta Neuropathol Commun. 2025. PMID: 40442822 Free PMC article.
-
18F PET with flutemetamol for the early diagnosis of Alzheimer's disease dementia and other dementias in people with mild cognitive impairment (MCI).Cochrane Database Syst Rev. 2017 Nov 22;11(11):CD012884. doi: 10.1002/14651858.CD012884. Cochrane Database Syst Rev. 2017. PMID: 29164602 Free PMC article.
Cited by
-
Organelle perturbation in Alzheimer's disease: do intracellular amyloid-ß and the fragmented Golgi mediate early intracellular neurotoxicity?Front Cell Dev Biol. 2025 Apr 15;13:1550211. doi: 10.3389/fcell.2025.1550211. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 40302938 Free PMC article. Review.
-
Advances in the cell biology of the trafficking and processing of amyloid precursor protein: impact of familial Alzheimer's disease mutations.Biochem J. 2024 Oct 2;481(19):1297-1325. doi: 10.1042/BCJ20240056. Biochem J. 2024. PMID: 39302110 Free PMC article. Review.
-
Detergent-free isolation and characterization of amyloid precursor protein C99 in E. coli native lipid-nanodiscs using non-ionic polymer.Protein Sci. 2025 Sep;34(9):e70276. doi: 10.1002/pro.70276. Protein Sci. 2025. PMID: 40828514 Free PMC article.
-
Association between renal function and memory-related disease: evidence from the China Health and Retirement Longitudinal Study.Ren Fail. 2025 Dec;47(1):2473668. doi: 10.1080/0886022X.2025.2473668. Epub 2025 Mar 4. Ren Fail. 2025. PMID: 40038268 Free PMC article.
-
Evaluating pathological levels of intracellular cholesterol through Raman and surface-enhanced Raman spectroscopies.Sci Rep. 2024 Nov 19;14(1):28566. doi: 10.1038/s41598-024-76621-5. Sci Rep. 2024. PMID: 39557950 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

