IL-1α is required for T cell-driven weight loss after respiratory viral infection
- PMID: 38382577
- PMCID: PMC11009121
- DOI: 10.1016/j.mucimm.2024.02.005
IL-1α is required for T cell-driven weight loss after respiratory viral infection
Abstract
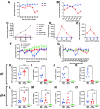
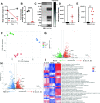
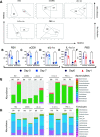
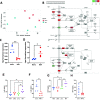
Respiratory viral infections remain a major cause of hospitalization and death worldwide. Patients with respiratory infections often lose weight. While acute weight loss is speculated to be a tolerance mechanism to limit pathogen growth, severe weight loss following infection can cause quality of life deterioration. Despite the clinical relevance of respiratory infection-induced weight loss, its mechanism is not yet completely understood. We utilized a model of CD 8+ T cell-driven weight loss during respiratory syncytial virus (RSV) infection to dissect the immune regulation of post-infection weight loss. Supporting previous data, bulk RNA sequencing indicated significant enrichment of the interleukin (IL)-1 signaling pathway after RSV infection. Despite increased viral load, infection-associated weight loss was significantly reduced after IL-1α (but not IL-1β) blockade. IL-1α depletion resulted in a reversal of the gut microbiota changes observed following RSV infection. Direct nasal instillation of IL-1α also caused weight loss. Of note, we detected IL-1α in the brain after either infection or nasal delivery. This was associated with changes in genes controlling appetite after RSV infection and corresponding changes in signaling molecules such as leptin and growth/differentiation factor 15. Together, these findings indicate a lung-brain-gut signaling axis for IL-1α in regulating weight loss after RSV infection.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

