Radiologic Classification of Hippocampal Sclerosis in Epilepsy
- PMID: 38383054
- PMCID: PMC11392372
- DOI: 10.3174/ajnr.A8214
Radiologic Classification of Hippocampal Sclerosis in Epilepsy
Abstract
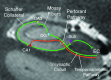
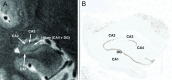
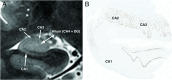
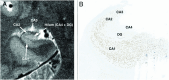
Temporal lobe epilepsy is a common form of epilepsy that is often associated with hippocampal sclerosis (HS). Although HS is commonly considered a binary assessment in radiologic evaluation, it is known that histopathologic changes occur in distinct clusters. Some subtypes of HS only affect certain subfields, resulting in minimal changes to the overall volume of the hippocampus. This is likely a major reason why whole hippocampal volumetrics have underperformed versus expert readers in the diagnosis of HS. With recent advancements in MRI technology, it is now possible to characterize the substructure of the hippocampus more accurately. However, this is not consistently addressed in radiographic evaluations. The histologic subtype of HS is critical for prognosis and treatment decision-making, necessitating improved radiologic classification of HS. The International League Against Epilepsy (ILAE) has issued a consensus classification scheme for subtyping HS histopathologic changes. This review aims to explore how the ILAE subtypes of HS correlate with radiographic findings, introduce a grading system that integrates radiologic and pathologic reporting in HS, and outline an approach to detecting HS subtypes by using MRI. This framework will not only benefit current clinical evaluations, but also enhance future studies involving high-resolution MRI in temporal lobe epilepsy.
© 2024 by American Journal of Neuroradiology.
Figures




References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
