Sensing the Spin State of Room-Temperature Switchable Cyanometallate Frameworks with Nitrogen-Vacancy Centers in Nanodiamonds
- PMID: 38383159
- PMCID: PMC10919078
- DOI: 10.1021/acsnano.3c11820
Sensing the Spin State of Room-Temperature Switchable Cyanometallate Frameworks with Nitrogen-Vacancy Centers in Nanodiamonds
Abstract
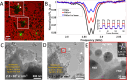
Room-temperature magnetically switchable materials play a vital role in current and upcoming quantum technologies, such as spintronics, molecular switches, and data storage devices. The increasing miniaturization of device architectures produces a need to develop analytical tools capable of precisely probing spin information at the single-particle level. In this work, we demonstrate a methodology using negatively charged nitrogen vacancies (NV-) in fluorescent nanodiamond (FND) particles to probe the magnetic switching of a spin crossover (SCO) metal-organic framework (MOF), [Fe(1,6-naphthyridine)2(Ag(CN)2)2] material (1), and a single-molecule photomagnet [X(18-crown-6)(H2O)3]Fe(CN)6·2H2O, where X = Eu and Dy (materials 2a and 2b, respectively), in response to heat, light, and electron beam exposure. We employ correlative light-electron microscopy using transmission electron microscopy (TEM) finder grids to accurately image and sense spin-spin interacting particles down to the single-particle level. We used surface-sensitive optically detected magnetic resonance (ODMR) and magnetic modulation (MM) of FND photoluminescence (PL) to sense spins to a distance of ca. 10-30 nm. We show that ODMR and MM sensing was not sensitive to the temperature-induced SCO of FeII in 1 as formation of paramagnetic FeIII through surface oxidation (detected by X-ray photoelectron spectroscopy) on heating obscured the signal of bulk SCO switching. We found that proximal FNDs could effectively sense the chemical transformations induced by the 200 keV electron beam in 1, namely, AgI → Ag0 and FeII → FeIII. However, transformations induced by the electron beam are irreversible as they substantially disrupt the structure of MOF particles. Finally, we demonstrate NV- sensing of reversible photomagnetic switching, FeIII + (18-crown-6) ⇆ FeII + (18-crown-6)+ •, triggered in 2a and 2b by 405 nm light. The photoredox process of 2a and 2b proved to be the best candidate for room-temperature single-particle magnetic switching utilizing FNDs as a sensor, which could have applications into next-generation quantum technologies.
Keywords: Nitrogen-vacancy sensing; metal−organic framework; nanodiamond; photomagnetism; spin-crossover; transmission electron microscopy.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






References
-
- Wang J. H.; Li Z. Y.; Yamashita M.; Bu X. H. Recent Progress on Cyano-bridged Transition-metal-based Single-Molecule Magnets and Single-chain Magnets. Coord. Chem. Rev. 2021, 428, 213617.10.1016/j.ccr.2020.213617. - DOI
LinkOut - more resources
Full Text Sources

