Risk factors for non-participation in ivermectin and dihydroartemisinin-piperaquine mass drug administration for malaria control in the MASSIV trial
- PMID: 38383367
- PMCID: PMC10882911
- DOI: 10.1186/s12936-024-04878-2
Risk factors for non-participation in ivermectin and dihydroartemisinin-piperaquine mass drug administration for malaria control in the MASSIV trial
Abstract
Background: Mass Drug Administration (MDA) has become a mainstay for the control of several diseases over the last two decades. Successful implementation of MDA programmes requires community participation and can be threatened by systematic non-participation. Such concerns are particularly pertinent for MDA programmes against malaria, as they require multi-day treatment over several consecutive months. Factors associated with non-participation to the MDA campaign with ivermectin (IVM) and dihydroartemisinin-piperaquine (DHP) implemented within the MASSIV cluster randomized trial were determined.
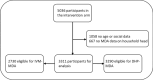
Methods: Coverage data was extracted from the MASSIV trial study database, with every datapoint being a directly observed therapy (DOT). A complete month of MDA was classified as receiving all three daily doses of treatment. For both ivermectin and DHP, ordinal logistic regression was used to identify individual and household level variables associated with non-participation.
Results: For ivermectin, 51.5% of eligible participants received all 3 months of treatment while 30.7% received either one or two complete months. For DHP, 56.7% of eligible participants received all 3 months of treatment and 30.5% received either one or two complete months. Children aged 5-15 years and adults aged more than 50 years were more likely to receive at least one complete month of MDA than working age adults, both for ivermectin (aOR 4.3, 95% CI 3.51-5.28 and aOR of 2.26, 95% CI 1.75-2.95) and DHP (aOR 2.47, 95%CI 2.02-3.02 and aOR 1.33, 95%CI 1.01-1.35), respectively. Members of households where the head received a complete month of MDA were more likely to themselves have received a complete month of MDA, both for ivermectin (aOR 1.71, 95%CI 1.35-2.14) and for DHP (aOR 1.64, 95%CI 1.33-2.04).
Conclusion: Personal and household-level variables were associated with participation in the MDA programme for malaria control. Specific strategies to (increase participation amongst some groups may be important to ensure maximum impact of MDA strategies in achieving malaria elimination.
Trial registration: The MASSIV trial is registered under NCT03576313.
Keywords: Ivermectin; MDA; Non-compliance.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Oswald WE, Kepha S, Halliday KE, Mcharo C, Safari T, Witek-McManus S, et al. Patterns of individual non-treatment during multiple rounds of mass drug administration for control of soil-transmitted helminths in the TUMIKIA trial, Kenya: a secondary longitudinal analysis. Lancet Glob Health. 2020;8:e1418–e1426. doi: 10.1016/S2214-109X(20)30344-2. - DOI - PMC - PubMed
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

