Ov-RPA-CRISPR/Cas12a assay for the detection of Opisthorchis viverrini infection in field-collected human feces
- PMID: 38383404
- PMCID: PMC10882828
- DOI: 10.1186/s13071-024-06134-7
Ov-RPA-CRISPR/Cas12a assay for the detection of Opisthorchis viverrini infection in field-collected human feces
Abstract
Background: Opisthorchis viverrini infection is traditionally diagnosed using the Kato-Katz method and formalin ethyl-acetate concentration technique. However, the limited sensitivity and specificity of these techniques have prompted the exploration of various molecular approaches, such as conventional polymerase chain reaction (PCR) and real-time PCR, to detect O. viverrini infection. Recently, a novel technique known as recombinase polymerase amplification (RPA)-clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein (Cas) (RPA-CRISPR/Cas) assay was developed as a point-of-care tool for the detection of various pathogens, including viruses and bacteria such as severe acute respiratory syndrome coronavirus 2 and Mycobacterium tuberculosis. This technology has demonstrated high sensitivity and specificity. Therefore, we developed and used the RPA-CRISPR/Cas assay to detect O. viverrini infection in field-collected human feces.
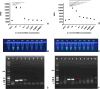
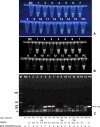
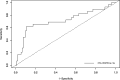
Methods: To detect O. viverrini infection in fecal samples, we developed a CRISPR/Cas12a (RNA-guided endonuclease) system combined with RPA (Ov-RPA-CRISPR/Cas12a). Several fecal samples, both helminth-positive and helminth-negative, were used for the development and optimization of amplification conditions, CRISPR/Cas detection conditions, detection limits, and specificity of the RPA-CRISPR/Cas12a assay for detecting O. viverrini infection. The detection results were determined using a real-time PCR system based on fluorescence values. Additionally, as the reporter was labeled with fluorescein, the detection results were visually inspected using an ultraviolet (UV) transilluminator. A receiver operating characteristic curve (ROC) was used to determine the optimal cutoff value for fluorescence detection. The diagnostic performance, including sensitivity and specificity, of the Ov-RPA-CRISPR/Cas12a assay was evaluated on the basis of comparison with standard methods.
Results: The Ov-RPA-CRISPR/Cas12a assay exhibited high specificity for detecting O. viverrini DNA. On the basis of the detection limit, the assay could detect O. viverrini DNA at concentrations as low as 10-1 ng using the real-time PCR system. However, in this method, visual inspection under UV light required a minimum concentration of 1 ng. To validate the Ov-RPA-CRISPR/Cas12a assay, 121 field-collected fecal samples were analyzed. Microscopic examination revealed that 29 samples were positive for O. viverrini-like eggs. Of these, 18 were confirmed as true positives on the basis of the Ov-RPA-CRISPR/Cas12a assay and microscopic examination, whereas 11 samples were determined as positive solely via microscopic examination, indicating the possibility of other minute intestinal fluke infections.
Conclusions: The Ov-RPA-CRISPR/Cas12a assay developed in this study can successfully detect O. viverrini infection in field-collected feces. Due to the high specificity of the assay reported in this study, it can be used as an alternative approach to confirm O. viverrini infection, marking an initial step in the development of point-of-care diagnosis.
Keywords: Clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein 12a (CRISPR/Cas12a); Diagnostic method; Fecal sample; Opisthorchiasis; Opisthorchis viverrini; Recombinase polymerase amplification.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- International Agency for Research on Cancer A review of human carcinogens: biological agents. IARC Monogr Eval Carcinog Risks Hum. 2012;100:341–370.
-
- Sripa B, Kaewkes S, Intapan PM, Maleewong W, Brindley PJ. Chapter 11. Food-borne trematodiases in Southeast Asia: epidemiology, pathology, clinical manifestation and control. Zhou X-N, Bergquist R, Olveda R, Utzinger J, editors. Adv Parasitol. 2010;72:p. 305–50. - PubMed
-
- Kopolrat KY, Singthong S, Khuntikeo N, Loilome W, Worasith C, Homwong C, et al. Performance of Mini Parasep® SF stool concentrator kit, Kato-Katz, and formalin-ethyl acetate concentration methods for diagnosis of opisthorchiasis in Northeast Thailand. Parasit Vectors. 2022;15:234. doi: 10.1186/s13071-022-05338-z. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

