Analysis of heterologous expression of phaCBA promotes the acetoin stress response mechanism in Bacillus subtilis using transcriptomics and metabolomics approaches
- PMID: 38383407
- PMCID: PMC10880289
- DOI: 10.1186/s12934-024-02334-z
Analysis of heterologous expression of phaCBA promotes the acetoin stress response mechanism in Bacillus subtilis using transcriptomics and metabolomics approaches
Abstract
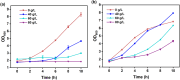
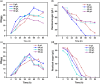
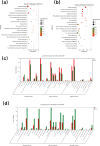
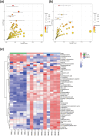
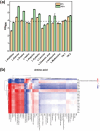
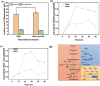
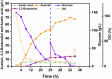
Acetoin, a versatile platform chemical and popular food additive, poses a challenge to the biosafety strain Bacillus subtilis when produced in high concentrations due to its intrinsic toxicity. Incorporating the PHB synthesis pathway into Bacillus subtilis 168 has been shown to significantly enhance the strain's acetoin tolerance. This study aims to elucidate the molecular mechanisms underlying the response of B. subtilis 168-phaCBA to acetoin stress, employing transcriptomic and metabolomic analyses. Acetoin stress induces fatty acid degradation and disrupts amino acid synthesis. In response, B. subtilis 168-phaCBA down-regulates genes associated with flagellum assembly and bacterial chemotaxis, while up-regulating genes related to the ABC transport system encoding amino acid transport proteins. Notably, genes coding for cysteine and D-methionine transport proteins (tcyB, tcyC and metQ) and the biotin transporter protein bioY, are up-regulated, enhancing cellular tolerance. Our findings highlight that the expression of phaCBA significantly increases the ratio of long-chain unsaturated fatty acids and modulates intracellular concentrations of amino acids, including L-tryptophan, L-tyrosine, L-leucine, L-threonine, L-methionine, L-glutamic acid, L-proline, D-phenylalanine, L-arginine, and membrane fatty acids, thereby imparting acetoin tolerance. Furthermore, the supplementation with specific exogenous amino acids (L-alanine, L-proline, L-cysteine, L-arginine, L-glutamic acid, and L-isoleucine) alleviates acetoin's detrimental effects on the bacterium. Simultaneously, the introduction of phaCBA into the acetoin-producing strain BS03 addressed the issue of insufficient intracellular cofactors in the fermentation strain, resulting in the successful production of 70.14 g/L of acetoin through fed-batch fermentation. This study enhances our understanding of Bacillus's cellular response to acetoin-induced stress and provides valuable insights for the development of acetoin-resistant Bacillus strains.
Keywords: Acetoin; Metabolomic; Polyhydroxybutyrate (PHB); Stress tolerance; Transcriptomic.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Roncal T, Caballero S, Díaz de Guereñu MDM, Rincón I, Prieto-Fernández S, Ochoa-Gómez JR. Efficient production of acetoin by fermentation using the newly isolated mutant strain Lactococcus lactis subsp. lactis CML B4. Process Biochem. 2017;58:35–41. doi: 10.1016/j.procbio.2017.04.007. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

