Sex-specific modulation of early life vocalization and cognition by Fmr1 gene dosage in a mouse model of Fragile X Syndrome
- PMID: 38383408
- PMCID: PMC10880250
- DOI: 10.1186/s13293-024-00594-3
Sex-specific modulation of early life vocalization and cognition by Fmr1 gene dosage in a mouse model of Fragile X Syndrome
Abstract
Background: Pup-dam ultrasonic vocalizations (USVs) are essential to cognitive and socio-emotional development. In autism and Fragile X Syndrome (FXS), disruptions in pup-dam USV communication hint at a possible connection between abnormal early developmental USV communication and the later emergence of communication and social deficits.
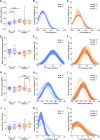
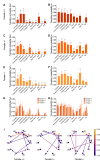
Methods: Here, we gathered USVs from PND 10 FXS pups during a short period of separation from their mothers, encompassing animals of all possible genotypes and both sexes (i.e., Fmr1-/y vs. Fmr1+/y males and Fmr1+/+, +/-, and -/- females). This allowed comparing the influence of sex and gene dosage on pups' communication capabilities. Leveraging DeepSqueak and analyzing vocal patterns, intricate vocal behaviors such as call structure, duration, frequency modulation, and temporal patterns were examined. Furthermore, homing behavior was assessed as a sensitive indicator of early cognitive development and social discrimination. This behavior relies on the use of olfactory and thermal cues to navigate and search for the maternal or nest odor in the surrounding space.
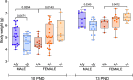
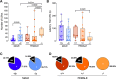
Results: The results show that FMRP-deficient pups of both sexes display an increased inclination to vocalize when separated from their mothers, and this behavior is accompanied by significant sex-specific changes in the main features of their USVs as well as in body weight. Analysis of the vocal repertoire and syntactic usage revealed that Fmr1 gene silencing primarily alters the USVs' qualitative composition in males. Moreover, sex-specific effects of Fmr1 silencing on locomotor activity and homing behavior were observed. FMRP deficiency in females increased activity, reduced nest-reaching time, and extended nest time. In males, it prolonged nest-reaching time and reduced nest time without affecting locomotion.
Conclusions: These findings highlight the interplay between Fmr1 gene dosage and sex in influencing communicative and cognitive skills during infancy.
Keywords: Autism spectrum disorder; Deep learning; Fragile X Syndrome; Homing behavior; Sex; Ultrasonic vocalizations.
Plain language summary
In this study, we investigated ultrasonic vocalizations (USVs) and homing behavior in a mouse model of Fragile X Syndrome (FXS), a leading genetic cause of autism spectrum disorder (ASD) caused by a mutation of the X-chromosome linked Fmr1 gene. Disruptions in pup-dam USV communication and cognitive skills may be linked to the later emergence of communication and social deficits in ASD. USVs were collected from 10-day-old FXS pups of all possible genotypes and both sexes during a short period of separation from their mothers. We utilized DeepSqueak, an advanced deep learning system, to examine vocal patterns and intricate vocal behaviors, including call structure, duration, frequency modulation, and their temporal patterns. Homing, a sensitive indicator of early cognitive development and social discrimination was assessed at P13. The results showed that FXS pups of both sexes displayed an increased inclination to vocalize when separated from their mothers. Examination of the vocal repertoire and its syntactic usage revealed that the silencing of the Fmr1 gene primarily alters the qualitative composition of ultrasonic communication in males. The sex-specific changes observed in USVs were accompanied by modifications in body weight. Regarding homing behavior, the deficiency of FMRP led to opposite deficits in activity, time to reach the nest, and nesting time depending on sex. Taken together, these findings highlight the interplay between Fmr1 gene dosage and sex in shaping communication and cognition during infancy.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Characterization of ultrasonic vocalizations of Fragile X mice.Behav Brain Res. 2016 Sep 1;310:76-83. doi: 10.1016/j.bbr.2016.04.016. Epub 2016 Apr 30. Behav Brain Res. 2016. PMID: 27142239
-
Reversal of ultrasonic vocalization deficits in a mouse model of Fragile X Syndrome with minocycline treatment or genetic reduction of MMP-9.Behav Brain Res. 2019 Oct 17;372:112068. doi: 10.1016/j.bbr.2019.112068. Epub 2019 Jul 2. Behav Brain Res. 2019. PMID: 31271818 Free PMC article.
-
Temporal and spectral differences in the ultrasonic vocalizations of fragile X knock out mice during postnatal development.Behav Brain Res. 2014 Feb 1;259:119-30. doi: 10.1016/j.bbr.2013.10.049. Epub 2013 Nov 7. Behav Brain Res. 2014. PMID: 24211451
-
FMR1 and Autism, an Intriguing Connection Revisited.Genes (Basel). 2021 Aug 6;12(8):1218. doi: 10.3390/genes12081218. Genes (Basel). 2021. PMID: 34440392 Free PMC article. Review.
-
Social behavior phenotypes in fragile X syndrome, autism, and the Fmr1 knockout mouse: theoretical comment on McNaughton et al. (2008).Behav Neurosci. 2008 Apr;122(2):483-9. doi: 10.1037/0735-7044.122.2.483. Behav Neurosci. 2008. PMID: 18410188 Review.
References
-
- Granon S, Faure A, Chauveau F, Cressant A, Ey E. Why should my mouse call me? Acoustic Communication in Mouse models of Social disorders: Ultrasonic vocalizations as an index of emotional and motivational States. Handb Behav Neurosci. 2018;25:423–31. doi: 10.1016/B978-0-12-809600-0.00040-8. - DOI
-
- Simola N, Granon S. Ultrasonic vocalizations as a tool in studying emotional states in rodent models of social behavior and brain disease. Neuropharmacology 2019;159. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

