Parkinson's disease-derived α-synuclein assemblies combined with chronic-type inflammatory cues promote a neurotoxic microglial phenotype
- PMID: 38383421
- PMCID: PMC10882738
- DOI: 10.1186/s12974-024-03043-5
Parkinson's disease-derived α-synuclein assemblies combined with chronic-type inflammatory cues promote a neurotoxic microglial phenotype
Abstract
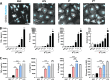
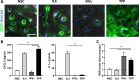
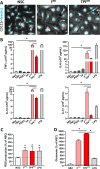
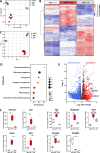
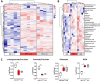
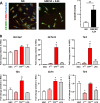
Parkinson's disease (PD) is a common age-related neurodegenerative disorder characterized by the aggregation of α-Synuclein (αSYN) building up intraneuronal inclusions termed Lewy pathology. Mounting evidence suggests that neuron-released αSYN aggregates could be central to microglial activation, which in turn mounts and orchestrates neuroinflammatory processes potentially harmful to neurons. Therefore, understanding the mechanisms that drive microglial cell activation, polarization and function in PD might have important therapeutic implications. Here, using primary microglia, we investigated the inflammatory potential of pure αSYN fibrils derived from PD patients. We further explored and characterized microglial cell responses to a chronic-type inflammatory stimulation combining PD patient-derived αSYN fibrils (FPD), Tumor necrosis factor-α (TNFα) and prostaglandin E2 (PGE2) (TPFPD). We showed that FPD hold stronger inflammatory potency than pure αSYN fibrils generated de novo. When combined with TNFα and PGE2, FPD polarizes microglia toward a particular functional phenotype departing from FPD-treated cells and featuring lower inflammatory cytokine and higher glutamate release. Whereas metabolomic studies showed that TPFPD-exposed microglia were closely related to classically activated M1 proinflammatory cells, notably with similar tricarboxylic acid cycle disruption, transcriptomic analysis revealed that TPFPD-activated microglia assume a unique molecular signature highlighting upregulation of genes involved in glutathione and iron metabolisms. In particular, TPFPD-specific upregulation of Slc7a11 (which encodes the cystine-glutamate antiporter xCT) was consistent with the increased glutamate response and cytotoxic activity of these cells toward midbrain dopaminergic neurons in vitro. Together, these data further extend the structure-pathological relationship of αSYN fibrillar polymorphs to their innate immune properties and demonstrate that PD-derived αSYN fibrils, TNFα and PGE2 act in concert to drive microglial cell activation toward a specific and highly neurotoxic chronic-type inflammatory phenotype characterized by robust glutamate release and iron retention.
Keywords: Chronic inflammation; Cystine/glutamate transporter; Iron metabolism; Metabolomic; Microglia; Neurotoxicity; Parkinson’s disease; Protein misfolding cyclic amplification; Transcriptomic; α-Synuclein.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that there are no conflicts of interest regarding the experimental part of this study.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

