Deep phenotyping of post-infectious myalgic encephalomyelitis/chronic fatigue syndrome
- PMID: 38383456
- PMCID: PMC10881493
- DOI: 10.1038/s41467-024-45107-3
Deep phenotyping of post-infectious myalgic encephalomyelitis/chronic fatigue syndrome
Abstract
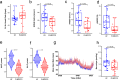
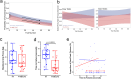
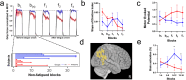
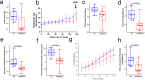
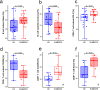
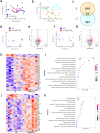
Post-infectious myalgic encephalomyelitis/chronic fatigue syndrome (PI-ME/CFS) is a disabling disorder, yet the clinical phenotype is poorly defined, the pathophysiology is unknown, and no disease-modifying treatments are available. We used rigorous criteria to recruit PI-ME/CFS participants with matched controls to conduct deep phenotyping. Among the many physical and cognitive complaints, one defining feature of PI-ME/CFS was an alteration of effort preference, rather than physical or central fatigue, due to dysfunction of integrative brain regions potentially associated with central catechol pathway dysregulation, with consequences on autonomic functioning and physical conditioning. Immune profiling suggested chronic antigenic stimulation with increase in naïve and decrease in switched memory B-cells. Alterations in gene expression profiles of peripheral blood mononuclear cells and metabolic pathways were consistent with cellular phenotypic studies and demonstrated differences according to sex. Together these clinical abnormalities and biomarker differences provide unique insight into the underlying pathophysiology of PI-ME/CFS, which may guide future intervention.
© 2024. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
MeSH terms
Substances
Grants and funding
- ZIA MH002922/ImNIH/Intramural NIH HHS/United States
- NS3157/U.S. Department of Health & Human Services | NIH | National Institute of Neurological Disorders and Stroke (NINDS)
- ZIA HL006210/ImNIH/Intramural NIH HHS/United States
- ZIA NS003157/ImNIH/Intramural NIH HHS/United States
- ZIA HL005199/ImNIH/Intramural NIH HHS/United States
- R01 DK071014/DK/NIDDK NIH HHS/United States
- SI2 AI138586/AI/NIAID NIH HHS/United States
- ZIA HL006212/ImNIH/Intramural NIH HHS/United States
- ZIC ES103362/ImNIH/Intramural NIH HHS/United States
- ES103362/U.S. Department of Health & Human Services | NIH | National Institute of Environmental Health Sciences (NIEHS)
- MH002922/U.S. Department of Health & Human Services | NIH | National Institute of Mental Health (NIMH)
- ZIA DK071014/ImNIH/Intramural NIH HHS/United States
- HL006210; HL006212; HL005199/U.S. Department of Health & Human Services | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- Z01 HL005199/ImNIH/Intramural NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

