Robot-assisted partial knee replacement versus standard total knee replacement (RoboKnees): a protocol for a pilot randomized controlled trial
- PMID: 38383530
- PMCID: PMC10880336
- DOI: 10.1186/s40814-024-01463-x
Robot-assisted partial knee replacement versus standard total knee replacement (RoboKnees): a protocol for a pilot randomized controlled trial
Abstract
Background: Total knee arthroplasty is a common surgery for end-stage knee osteoarthritis. Partial knee arthroplasty is also a treatment option for patients with arthritis present in only one or two knee compartments. Partial knee arthroplasty can preserve the natural knee biomechanics, but these replacements may not last as long as total knee replacements. Robotic-assisted orthopedic techniques can help facilitate partial knee replacements, increasing accuracy and precision. This trial will investigate the feasibility and assess clinical outcomes for a larger definitive trial.
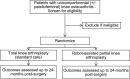
Methods: This is a protocol for an ongoing parallel randomized pilot trial of 64 patients with uni- or bicompartmental knee arthritis. Patients are randomized to either receive robot-assisted partial knee arthroplasty or manual total knee arthroplasty. The primary outcome of this pilot is investigating the feasibility of a larger trial. Secondary (clinical) outcomes include joint awareness, return to activities, knee function, patient global impression of change, persistent post-surgical pain, re-operations, resource utilization and cost-effectiveness, health-related quality of life, radiographic alignment, knee kinematics during walking gait, and complications up to 24 months post-surgery.
Discussion: The RoboKnees pilot study is the first step in determining the outcome of robot-assisted partial knee replacements. Conclusions from this study will be used to design future large-scale trials. This study will inform surgeons about the potential benefits of robot-assisted partial knee replacements.
Trial registration: This study was prospectively registered on clinicaltrials.gov (identifier: NCT04378049) on 4 May 2020, before the first patient was randomized.
Keywords: Feasibility; Knee replacement; Protocol; Randomized controlled trial; Robot-assisted surgery.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
References
-
- Canada S. Table 13-10-0096-06 Health characteristics, annual estimates. 2022.
-
- Information, CIfH, Hip and Knee Replacements in Canada: CJRR Quick Stats, 2019–2020. 2021: Ottawa, ON.
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical