Immunogenicity phase II study evaluating booster capacity of nonadjuvanted AKS-452 SARS-Cov-2 RBD Fc vaccine
- PMID: 38383578
- PMCID: PMC10881471
- DOI: 10.1038/s41541-024-00830-2
Immunogenicity phase II study evaluating booster capacity of nonadjuvanted AKS-452 SARS-Cov-2 RBD Fc vaccine
Abstract
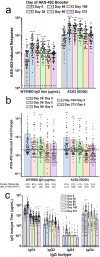
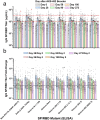
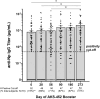
AKS-452, a subunit vaccine comprising an Fc fusion of the ancestral wild-type (WT) SARS-CoV-2 virus spike protein receptor binding domain (SP/RBD), was evaluated without adjuvant in a single cohort, non-randomized, open-labelled phase II study (NCT05124483) at a single site in The Netherlands for safety and immunogenicity. A single 90 µg subcutaneous booster dose of AKS-452 was administered to 71 adults previously primed with a registered mRNA- or adenovirus-based vaccine and evaluated for 273 days. All AEs were mild and no SAEs were attributable to AKS-452. While all subjects showed pre-existing SP/RBD binding and ACE2-inhibitory IgG titers, 60-68% responded to AKS-452 via ≥2-fold increase from days 28 to 90 and progressively decreased back to baseline by day 180 (days 28 and 90 mean fold-increases, 14.7 ± 6.3 and 8.0 ± 2.2). Similar response kinetics against RBD mutant proteins (including omicrons) were observed but with slightly reduced titers relative to WT. There was an expected strong inverse correlation between day-0 titers and the fold-increase in titers at day 28. AKS-452 enhanced neutralization potency against live virus, consistent with IgG titers. Nucleocapsid protein (Np) titers suggested infection occurred in 66% (46 of 70) of subjects, in which only 20 reported mild symptomatic COVID-19. These favorable safety and immunogenicity profiles support booster evaluation in a planned phase III universal booster study of this room-temperature stable vaccine that can be rapidly and inexpensively manufactured to serve vaccination at a global scale without the need of a complex distribution or cold chain.
© 2024. The Author(s).
Conflict of interest statement
The following authors declare the following financial interests/personal relationships which may be considered as potential competing interests: the following authors were employed by and received monetary compensation from Akston Biosciences, Inc.: DGA, ARD, MMS, SM, RR, EKG, TS, SK, JRH, NJS, VR, SN, TML, TZ. No other authors were personally compensated by Akston Biosciences.
Figures




References
LinkOut - more resources
Full Text Sources
Miscellaneous

