Targeting PI3K/Akt/mTOR signaling in rodent models of PMP22 gene-dosage diseases
- PMID: 38383802
- PMCID: PMC10940316
- DOI: 10.1038/s44321-023-00019-5
Targeting PI3K/Akt/mTOR signaling in rodent models of PMP22 gene-dosage diseases
Abstract
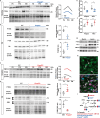
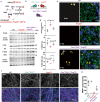
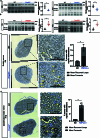
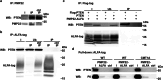
Haplo-insufficiency of the gene encoding the myelin protein PMP22 leads to focal myelin overgrowth in the peripheral nervous system and hereditary neuropathy with liability to pressure palsies (HNPP). Conversely, duplication of PMP22 causes Charcot-Marie-Tooth disease type 1A (CMT1A), characterized by hypomyelination of medium to large caliber axons. The molecular mechanisms of abnormal myelin growth regulation by PMP22 have remained obscure. Here, we show in rodent models of HNPP and CMT1A that the PI3K/Akt/mTOR-pathway inhibiting phosphatase PTEN is correlated in abundance with PMP22 in peripheral nerves, without evidence for direct protein interactions. Indeed, treating DRG neuron/Schwann cell co-cultures from HNPP mice with PI3K/Akt/mTOR pathway inhibitors reduced focal hypermyelination. When we treated HNPP mice in vivo with the mTOR inhibitor Rapamycin, motor functions were improved, compound muscle amplitudes were increased and pathological tomacula in sciatic nerves were reduced. In contrast, we found Schwann cell dedifferentiation in CMT1A uncoupled from PI3K/Akt/mTOR, leaving partial PTEN ablation insufficient for disease amelioration. For HNPP, the development of PI3K/Akt/mTOR pathway inhibitors may be considered as the first treatment option for pressure palsies.
Keywords: Charcot–Marie–Tooth Neuropathies; Myelin; PI3K/Akt/mTOR Signaling; Peripheral Myelin Protein of 22 kDa; Schwann Cell.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures








References
-
- Adlkofer K, Martini R, Aguzzi A, Zielasek J, Toyka KV, Suter U. Hypermyelination and demyelinating peripheral neuropathy in Pmp22-deficient mice. Nat Genet. 1995;11:274–280. - PubMed
-
- Archelos JJ, Roggenbuck K, Schneider-Schaulies J, Linington C, Toyka KV, Hartung HP. Production and characterization of monoclonal antibodies to the extracellular domain of P0. J Neurosci Res. 1993;35:46–53. - PubMed
-
- Attarian S, Fatehi F, Rajabally YA, Pareyson D. Hereditary neuropathy with liability to pressure palsies. J Neurol. 2020;267:2198–2206. - PubMed
-
- Azzedine H, Bolino A, Taieb T, Birouk N, Di Duca M, Bouhouche A, Benamou S, Mrabet A, Hammadouche T, Chkili T, et al. Mutations in MTMR13, a new pseudophosphatase homologue of MTMR2 and Sbf1, in two families with an autosomal recessive demyelinating form of Charcot-Marie-Tooth disease associated with early-onset glaucoma. Am J Hum Genet. 2003;72:1141–1153. - PMC - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

