Another renaissance for bile acid gastrointestinal microbiology
- PMID: 38383804
- PMCID: PMC11558780
- DOI: 10.1038/s41575-024-00896-2
Another renaissance for bile acid gastrointestinal microbiology
Abstract
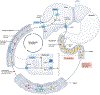
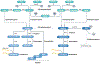
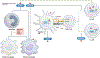
The field of bile acid microbiology in the gastrointestinal tract is going through a current rebirth after a peak of activity in the late 1970s and early 1980s. This renewed activity is a result of many factors, including the discovery near the turn of the century that bile acids are potent signalling molecules and technological advances in next-generation sequencing, computation, culturomics, gnotobiology, and metabolomics. We describe the current state of the field with particular emphasis on questions that have remained unanswered for many decades in both bile acid synthesis by the host and metabolism by the gut microbiota. Current knowledge of established enzymatic pathways, including bile salt hydrolase, hydroxysteroid dehydrogenases involved in the oxidation and epimerization of bile acid hydroxy groups, the Hylemon-Bjӧrkhem pathway of bile acid C7-dehydroxylation, and the formation of secondary allo-bile acids, is described. We cover aspects of bile acid conjugation and esterification as well as evidence for bile acid C3-dehydroxylation and C12-dehydroxylation that are less well understood but potentially critical for our understanding of bile acid metabolism in the human gut. The physiological consequences of bile acid metabolism for human health, important caveats and cautionary notes on experimental design and interpretation of data reflecting bile acid metabolism are also explored.
© 2024. Springer Nature Limited.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures


Similar articles
-
The Hylemon-Björkhem pathway of bile acid 7-dehydroxylation: history, biochemistry, and microbiology.J Lipid Res. 2023 Aug;64(8):100392. doi: 10.1016/j.jlr.2023.100392. Epub 2023 May 19. J Lipid Res. 2023. PMID: 37211250 Free PMC article. Review.
-
Interactions between gut bacteria and bile in health and disease.Mol Aspects Med. 2017 Aug;56:54-65. doi: 10.1016/j.mam.2017.06.002. Epub 2017 Jun 21. Mol Aspects Med. 2017. PMID: 28602676 Review.
-
Metabolism of Oxo-Bile Acids and Characterization of Recombinant 12α-Hydroxysteroid Dehydrogenases from Bile Acid 7α-Dehydroxylating Human Gut Bacteria.Appl Environ Microbiol. 2018 May 1;84(10):e00235-18. doi: 10.1128/AEM.00235-18. Print 2018 May 15. Appl Environ Microbiol. 2018. PMID: 29549099 Free PMC article.
-
Consequences of bile salt biotransformations by intestinal bacteria.Gut Microbes. 2016;7(1):22-39. doi: 10.1080/19490976.2015.1127483. Gut Microbes. 2016. PMID: 26939849 Free PMC article. Review.
-
Metabolism of cholesterol and bile acids by the gut microbiota.Pathogens. 2013 Dec 30;3(1):14-24. doi: 10.3390/pathogens3010014. Pathogens. 2013. PMID: 25437605 Free PMC article.
Cited by
-
Ileal microbial microbiome and its secondary bile acids modulate susceptibility to nonalcoholic steatohepatitis in dairy goats.Microbiome. 2024 Nov 23;12(1):247. doi: 10.1186/s40168-024-01964-0. Microbiome. 2024. PMID: 39578870 Free PMC article.
-
HLF and PPARα axis regulates metabolic-associated fatty liver disease through extracellular vesicles derived from the intestinal microbiota.Imeta. 2025 Apr 7;4(2):e70022. doi: 10.1002/imt2.70022. eCollection 2025 Apr. Imeta. 2025. PMID: 40236774 Free PMC article.
-
Crosstalk between bile acids and gut microbiota: a potential target for precancerous lesions of gastric cancer.Front Pharmacol. 2025 Mar 13;16:1533141. doi: 10.3389/fphar.2025.1533141. eCollection 2025. Front Pharmacol. 2025. PMID: 40183085 Free PMC article. Review.
-
Host-Microbial Cometabolite Ursodeoxycholic Acid Protects Against Poststroke Cognitive Impairment.J Am Heart Assoc. 2025 May 6;14(9):e038862. doi: 10.1161/JAHA.124.038862. Epub 2025 Apr 23. J Am Heart Assoc. 2025. PMID: 40265603 Free PMC article.
-
Phase I trial comparing bile acid and short-chain fatty acid alterations in stool collected from human subjects treated with omadacycline or vancomycin.Antimicrob Agents Chemother. 2025 Feb 13;69(2):e0125124. doi: 10.1128/aac.01251-24. Epub 2025 Jan 17. Antimicrob Agents Chemother. 2025. PMID: 39819014 Free PMC article. Clinical Trial.
References
-
- Haslewood GA Bile salt evolution. J Lipid Res 8, 535–550 (1967). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

