Daprodustat and Heart Failure in CKD
- PMID: 38383961
- PMCID: PMC11149037
- DOI: 10.1681/ASN.0000000000000321
Daprodustat and Heart Failure in CKD
Abstract
Key Points:
Patients with CKD face meaningful risk of heart failure hospitalization.
Daprodustat compared with darbepoetin was associated with a nonsignificantly greater number of heart failure hospitalizations in non-dialysis patients.
Background: Patients with CKD are at higher risk of heart failure. The hypoxia-inducible factor prolyl hydroxylase inhibitor daprodustat is an orally acting alternative to conventional injectable erythropoietin-stimulating agents (ESAs) for the treatment of anemia in patients with CKD. Whether daprodustat affects the risk of heart failure hospitalization is unknown.
Methods: The Anemia Studies in Chronic Kidney Disease: Erythropoiesis via a Novel Prolyl Hydroxylase Inhibitor Daprodustat–Dialysis (ASCEND-D; n=2964) and Anemia Studies in Chronic Kidney Disease: Erythropoiesis via a Novel Prolyl Hydroxylase Inhibitor Daprodustat–Non-Dialysis (ASCEND-ND; n=3872) trials compared daprodustat with conventional ESA in patients with anemia of CKD who did or did not require dialysis, respectively. We identified risk factors of heart failure hospitalization and assessed the effect of daprodustat compared with conventional ESA on heart failure hospitalizations.
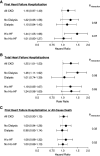
Results: History of heart failure, diabetes, and higher systolic BP were independently associated with heart failure hospitalization in both trials, irrespective of treatment assignment. The number of first heart failure hospitalizations was greater in the daprodustat arm in patients not receiving dialysis (hazard ratio [HR], 1.22 [95% confidence interval (CI), 0.95 to 1.56], P = 0.12) and in patients receiving dialysis (HR, 1.10 [95% CI, 0.84 to 1.45], P = 0.47), although these differences were not statistically significant. HRs in patients with and without history of heart failure were 1.37 (95% CI, 0.89 to 2.11) versus 1.08 (95% CI, 0.79 to 1.46) (P-interaction=0.36) in the ASCEND-ND trial and 1.52 (95% CI, 0.97 to 2.38) versus 0.93 (95% CI, 0.66 to 1.30) (P-interaction=0.09) in the ASCEND-D trial, respectively. In post hoc analyses, daprodustat increased total (first and recurrent) heart failure hospitalizations in participants not receiving dialysis (rate ratio, 1.46 [95% CI, 1.11 to 1.92], P = 0.007) but not in participants receiving dialysis (rate ratio, 1.01 [95% CI, 0.74 to 1.39], P = 0.93). Daprodustat did not significantly affect the risk of a composite outcome of first heart failure hospitalization or death.
Conclusions: A greater number of first heart failure hospitalization events occurred in patients treated with daprodustat compared with conventional ESA, but this difference did not reach statistical significance. Differences in the number of heart failure hospitalization events were most apparent in patients not receiving dialysis and in patients with history of heart failure.
Podcast:
This article contains a podcast at
Trial registration: ClinicalTrials.gov NCT02879305 NCT02876835.
Conflict of interest statement
K. Carroll reports employment with KJC Statistics Limited; consultancy fees from Cerium Pharmaceuticals Inc., Chinook Therapeutics Inc., Dimerix, GSK, Omeros Corp., Reata Pharmaceuticals, Sanifit Therapeutics, and Travere Therapeutics Inc., as a part of KJC Statistics Limited with ownership interests; ownership interest in KJC Statistics Limited; and advisory or leadership roles as a GSK Steering Committee Member and Omeros Academic Advisory Board. B.L. Claggett reports consultancy for Alnylam, Bristol Myers Squibb, Cardior, Cardurion, Corvia, Cytokinetics, CVRx, Intellia, Novartis, and Rocket and advisory or leadership role for Eli Lilly. J.W. Cunningham reports consultancy for Edgewise Therapeutics, KCK, Occlutech, Roche Diagnostics, and grant support from the American Heart Association (23CDA1052151) and Harvard Catalyst. T. Hiemstra reports employment with GlaxoSmithKline; ownership interest in GlaxoSmithKline, Kodika Corporation, and Ovibio Corporation; and advisory or leadership roles as SAB Member of the International Clinical Trial Center Network and Steering Board Member of the PHOSPHATE Trial. K. Khavandi reports employment with GSK, ownership interest in BenevolentAI and GSK, and advisory or leadership roles for BenevolentAI and GSK. R.D. Lopes reports consultancy for AstraZeneca, Bayer, Boehringer Ingelgeim, Bristol Myers Squibb, and Novo Nordisk; research funding from Amgen, Bristol Myers Squibb, GlaxoSmithKline, Medtronic, Pfizer, and Sanofi-Aventis; funding for educational activities or lectures from Daiichi Sankyo, Novo Nordisk, and Pfizer; and honoraria from AstraZeneca, Daiichi Sankyo, Novo Nordisk, and Pfizer. M.A. Lukas reports employment with GlaxoSmithKline; consultancy for Abbott FFS, Encore Medical Inc., and Medtronic; and ownership interest in GSK. J.J.V. McMurray reports employment with Glasgow University; personal consultancy fees from Alynylam Pharmaceuticals, Bayer, BMS, Ionis Pharmaceuticals, Novartis, Regeneron Pharmaceuticals, and River 2 Renal Corp.; role on Data Safety Monitoring Board for George Clinical PTY Ltd.; and personal lecture fees from Abbott, Alkem Metabolics, AZ, BI, Blue Ocean Scientific Solutions Ltd., Canadian Medical and Surgical Knowledge, Emcure Pharma., Eris Lifesciences, European Academy of CME, Global Clinical Trial Partners (GCTP), Hikma Pharma., Imagica Health, Intas Pharma., J.B. Chemicals & Pharma., Lupin Pharma., Medscape/Heart.Org., ProAdWise Communications, Radcliffe Cardiology, Servier Director, Sun Pharma., The Corpus, Translation Research Group, and Translational Medicine Academy. J.J.V. McMurray's employer, Glasgow University, has been paid for J.J.V. McMurray's participation in advisory boards organized by AstraZeneca and Novartis. J.J.V. McMurray reports payments to employer, Glasgow University, for work on clinical trials, consulting, and other activities for Amgen, AstraZeneca, Bayer, Cardurion, Cytokinetics, GSK, KBP Biosciences, and Novartis and as Director of Global Clinical Trial Partners Ltd. (GCTP). J.J.V. McMurray has received payments through Glasgow University for work on clinical trials, consulting, and other activities from Alnylam, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, BMS, Cardurion, Cytokinetics, Dal-Cor, GSK, Ionis, KBP Biosciences, Novartis, Pfizer, and Theracos. V. Perkovic reports consultancy for AbbVie, AstraZeneca, Baxter, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Chinook, Durect, Eli Lilly, Gilead, GlaxoSmithKline, Janssen, Merck, Mitsubishi Tanabe, Mundipharma, Novartis, Novo Nordisk, Otsuka, Pfizer, Pharmalink, Relypsa, Retrophin, Roche, Sanofi, Servier, Travere, Tricida, UpToDate, and Vitae; ownership interest in George Clinical; research funding from AstraZeneca, Bayer, Chinook, Gilead, GlaxoSmithKline, Janssen, Novartis, Novo Nordisk, Otsuka, Pfizer (supplied drug and seed funding for TESTING trial), Travere, and Tricida; honoraria from AbbVie, Astellas, AstraZeneca, Baxter, Bayer, Boehringer Ingelheim, Chinook, Durect, Eli Lilly, Gilead, GlaxoSmithKline, Janssen, Merck, Mitsubishi Tanabe, Mundipharma, Novartis, Novo Nordisk, Otsuka, Pfizer, Pharmalink, Relypsa, Retrophin, Roche, Sanofi, Servier, Travere, Tricida, UpToDate, and Vitae; serving/served on Steering Committees for trials funded by AbbVie, AstraZeneca, Bayer, Boehringer Ingelheim, Chinook, Eli Lilly, Gilead, GSK, Janssen, Novartis, Novo Nordisk, and Retrophin; and advisory or leadership roles for Steering committees for Bayer, Chinook, GlaxoSmithKline, Janssen, Novartis, Novo Nordisk, Otsuka, Pfizer, and Travere; and role as Board Director for George Clinical and St Vincents Health Australia. V. Perkovic reports other interests/relationships: Board Director: Childrens Cancer Institute, George Clinical, George Institute, Garvan Institute, Mindgardens Network, and Victor Chang Cardiac Research Institute. P. Ranganathan reports employment with GlaxoSmithKline and ownership interest in AMD—advanced micro devices and SQ—block Inc. J. Shannon reports employment with GlaxoSmithKline and ownership interest in GlaxoSmithKline and Syneos Health. A.K. Singh reports consultancy for Bayer, Chinook/Novartis, GSK,
Figures



References
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

