Hexasodium fytate exposure-response correlations in a randomized, placebo-controlled study of patients on dialysis with cardiovascular calcification
- PMID: 38384289
- PMCID: PMC10879272
- DOI: 10.3389/fphar.2024.1325186
Hexasodium fytate exposure-response correlations in a randomized, placebo-controlled study of patients on dialysis with cardiovascular calcification
Abstract
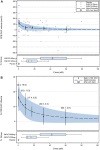
Background: Patients receiving dialysis have high cardiovascular risk in part due to extensive vascular calcification. In the CaLIPSO study, infusion of hexasodium fytate (SNF472), the hexasodium salt of inositol hexaphosphate, for 52 weeks thrice weekly during hemodialysis significantly reduced progression of coronary artery calcification (CAC). This report examines pharmacokinetic/pharmacodynamic (PK/PD) and exposure-efficacy in CaLIPSO. Methods: We measured hexasodium fytate plasma concentrations (PK) by validated liquid chromatography-mass spectroscopy, and hydroxyapatite crystallization in plasma (PD) by validated spectrophotometry. Analyses included patients evaluable for PK, PD, and CAC change (per-protocol analysis). We developed a simple Emax model for maximum concentration (Cmax) and PD effect, and linear and non-linear Emax models for exposure-efficacy among individual average Cmax and absolute and percent changes in CAC score from baseline to week 52. Results: Among evaluable patients receiving placebo (n = 15), 300 mg (n = 20), or 600 mg (n = 20), average Cmax across visits was not quantifiable (<0.76 μM), 15 μM, and 46 μM, respectively. These results suggest a more-than-proportional increase, without accumulation, with a Cmax ratio of approximately 3 for the doses administered. Average inhibition of hydroxyapatite crystallization was 15%, 61%, and 75%, respectively, and similar across visits. Simple Emax models described 80% maximal effect at exposures >21.9 µM and a plateau in exposure-efficacy above the third quartile of Cmax (≥32 µM). Conclusion: Hexasodium fytate has exposure-dependent effects on hydroxyapatite crystallization and progression of cardiovascular calcification. Simple Emax models show robust relations among exposure, inhibition of hydroxyapatite crystallization, and change in CAC volume. Clinical Trial Registration: https://www.clinicaltrials.gov; identifier NCT02966028.
Keywords: SNF472; calcification; cardiovascular; hexasodium fytate; pharmacodynamics; pharmacokinetics.
Copyright © 2024 Perelló, Alberti, Torres, Ferrer, Perez, Bassissi, Gold, Raggi, Chertow and Salcedo.
Conflict of interest statement
JP, FB, and MF are consultants for Sanifit and have patents related to hexasodium fytate. MP and CS are employees of Sanifit and have patents related to hexasodium fytate. JA, JT, and PR have served as consultants to Sanifit. JT is employed by Optimapharm. AG was an employee of Sanifit and has patents related to hexasodium fytate. GC serves on the Board of Directors for Satellite Healthcare, a non-profit dialysis provider; has received research grants from NIDDK, NIAID, and CSL Behring; has served on trial steering committees with Akebia, AstraZeneca, Gilead, Sanifit, and Vertex; has served as an advisor to Ardelyx, CloudCath, Durect, Miromatrix, Outset, Renibus, Reata, and Unicycive; and has served on data safety monitoring boards for NIDDK, Bayer, Mineralys, and ReCor.
Figures



References
-
- Bellasi A., Raggi P., Bover J., Bushinsky D. A., Chertow G. M., Ketteler M., et al. (2021). Trial design and baseline characteristics of CaLIPSO: a randomized, double-blind placebo-controlled trial of SNF472 in patients receiving haemodialysis with cardiovascular calcification. Clin. Kidney J. 14 (1), 366–374. 10.1093/ckj/sfz144 - DOI - PMC - PubMed
-
- Brandenburg V. M., Sinha S., Torregrosa J. V., Garg R., Miller S., Canals A. Z., et al. (2019). Improvement in wound healing, pain, and quality of life after 12 weeks of SNF472 treatment: a phase 2 open-label study of patients with calciphylaxis. J. Nephrol. 32 (5), 811–821. 10.1007/s40620-019-00631-0 - DOI - PubMed
-
- Bushinsky D. A., Raggi P., Bover J., Ketteler M., Bellasi A., Rodriguez M., et al. (2021). Effects of myo-inositol hexaphosphate (SNF472) on bone mineral density in patients receiving hemodialysis: an analysis of the randomized, placebo-controlled CaLIPSO study. Clin. J. Am. Soc. Nephrol. 16 (5), 736–745. 10.2215/CJN.16931020 - DOI - PMC - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical

