Effects of tofersen treatment in patients with SOD1-ALS in a "real-world" setting - a 12-month multicenter cohort study from the German early access program
- PMID: 38384337
- PMCID: PMC10878861
- DOI: 10.1016/j.eclinm.2024.102495
Effects of tofersen treatment in patients with SOD1-ALS in a "real-world" setting - a 12-month multicenter cohort study from the German early access program
Abstract
Background: In April 2023, the antisense oligonucleotide tofersen was approved by the U.S. Food and Drug Administration (FDA) for treatment of SOD1-amyotrophic lateral sclerosis (ALS), after a decrease of neurofilament light chain (NfL) levels had been demonstrated.
Methods: Between 03/2022 and 04/2023, 24 patients with SOD1-ALS from ten German ALS reference centers were followed-up until the cut-off date for ALS functional rating scale revised (ALSFRS-R), progression rate (loss of ALSFRS-R/month), NfL, phosphorylated neurofilament heavy chain (pNfH) in cerebrospinal fluid (CSF), and adverse events.
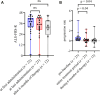
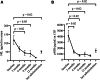
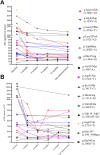
Findings: During the observation period, median ALSFRS-R decreased from 38.0 (IQR 32.0-42.0) to 35.0 (IQR 29.0-42.0), corresponding to a median progression rate of 0.11 (IQR -0.09 to 0.32) points of ALSFRS-R lost per month. Median serum NfL declined from 78.0 pg/ml (IQR 37.0-147.0 pg/ml; n = 23) to 36.0 pg/ml (IQR 22.0-65.0 pg/ml; n = 23; p = 0.02), median pNfH in CSF from 2226 pg/ml (IQR 1061-6138 pg/ml; n = 18) to 1151 pg/ml (IQR 521-2360 pg/ml; n = 18; p = 0.02). In the CSF, we detected a pleocytosis in 73% of patients (11 of 15) and an intrathecal immunoglobulin synthesis (IgG, IgM, or IgA) in 9 out of 10 patients. Two drug-related serious adverse events were reported.
Interpretation: Consistent with the VALOR study and its Open Label Extension (OLE), our results confirm a reduction of NfL serum levels, and moreover show a reduction of pNfH in CSF. The therapy was safe, as no persistent symptoms were observed. Pleocytosis and Ig synthesis in CSF with clinical symptoms related to myeloradiculitis in two patients, indicate the potential of an autoimmune reaction.
Funding: No funding was received towards this study.
Keywords: Amyotrophic lateral sclerosis; Antisense oligonucleotide; Early access program; SOD1; Tofersen.
© 2024 The Author(s).
Conflict of interest statement
MW reports no competing interests. JD reports speaker honoraria from Biogen Inc. ZE, ÖP, KK, KM, UWei, CH, JS, KM, RS, FB, TS, KG, EF, AK, TK, PR, UWey, MV, MJ, THaa, IV, JH, JC, JHW, PS, PK, WPR and SW report no competing interests. DB reports stocks from Ionis Pharmaceuticals (100 stocks). ZU reports financial support from Biogen, Roche and Novartis for SMArtCARE data collection (site Ulm). He received grants, consulting fees for advisory boards, lectures, presentations, manuscript writing, educational events, and support for travel expenses from Biogen. He received payment for expert testimony (Case report, no guideline development) from Thieme and Biogen. AF reports grants or contracts from DFG (German Research Foundation, Project funding not related to this manuscript). MR received lecture honoraria from Zambon. AG reports grants from German Society of Cryobanks (GDK e.V.). SP reports grants or contracts from the German Israeli Foundation, the German Neuromuscular Society, and the Neurodegenerative Research Inc. SP reports consulting fees from Amylyx, Biogen, ITF Pharma, Roche, Zambon and Ferrer, and payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Amylyx, Biogen, ITF Pharma, Roche, and Zambon. SP received support for travel and/or attend meetings from Amylyx, Zambon, and PTC Therapeutics. JG reports a startup grant from the University foundation of the University of Lübeck. He received consultation fees from Amylyx, Clene Nanomedicine for consultancy on drug development in ALS and payment for expert testimony on EU and national level from Amylyx. JG participated on Data Safety Monitoring Boards or Advisory Boards for UCB, ITF Pharma, and Ferrer. FS reports participation on Advisory Boards for Amylyx, Alnylam, and Alexion. THag reports grants and honoraria from Biogen, Roche, Novartis (all for SMA-Research), Sanofi Genzyme (Neuromuscular Diseases Research), and payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Alexion, Argenx (both for Myasthenia gravis), Biogen, Novartis, Sanofi Genzyme, Roche (SMA) and Amylyx (ALS). THag participated on Data Safety Monitoring Boards or Advisory Boards for Biogen, Novartis, Roche (all for SMA), Alexion, Argenx, UCB (all for Myasthenia gravis), Amylyx (for ALS), Sanofi Genzyme (for LOPD). RG reports honoraria for lectures from Biogen, Roche, and Zambon. He participated on Advisory Boards for Biogen, Roche, Zambon, and ITF Pharma. RG reports research support from Biogen and Zambon. PW served on Advisory Boards for Zambon, and ITF Pharma. PK reports consulting fees from Biogen. TM is on the Advisory Board of Biogen and received consulting fees from Biogen. MS reports consulting fees from Alexion, Bayer, Biogen, Bristol-Myers-Squibb/Celgene, Merck, Horizon, Roche, and Sanofi Genzyme. MS reports payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Alexion, Horizon, Roche, and Sanofi Genzyme and support for attending meetings and/or travel from Alexion, Celgene, Horizon, Roche, and Sanofi Genzyme. MS participated on Data Safety Monitoring Board or Advisory Board for Alexion, Bayer, Biogen, Bristol-Myers-Squibb, Merck, Horizon, Roche, and Sanofi Genzyme. HT reports grants or contracts from Sanofi Genzyme, German Multiple Sclerosis Society (DMSG), AMSEL Ursula-Späth-Stiftung, Bayern-DMSG, Deutsche MS-Stiftung, Ministry of Science, Research and Arts of the State Baden-Württemberg (MWK-BW), Chemische Fabrik Karl Bucher. HT received consulting fees from Merck, Novartis, Roche and received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Alexion, Bayer, Biogen, Celgene, GSK, Janssen, Merck, Novartis, Roche, Sanofi Genzyme, Siemens, TEVA, Viatris. HT reports support for attending meetings and/or travel from Janssen, Merck, Novartis, Roche, Sanofi Genzyme. He reports leadership or fiduciary role in DGLN, DMSG and AMSEL. ACL is a member of Advisory Boards of Roche Pharma AG, Biogen, Alector and Amylyx. He received compensation for talks from Biologix, the German Society of Neurology, Biogen, Springer Medicine, Amylyx and the company Streamed Up! GmbH. He is involved in trials which are sponsored by Amylyx, Ferrer International, Novartis Research and Development, Mitsubishi Tanabe, Apellis Pharmaceuticals, Alexion, Orion Pharma, the European Union, BMBF, Biogen and Orphazyme, Ionis Pharmaceuticals, QurAlis and Alector.
Figures


References
LinkOut - more resources
Full Text Sources
Miscellaneous

