T follicular helper cells expansion in transplant recipients correlates with graft infiltration and adverse outcomes
- PMID: 38384450
- PMCID: PMC10879567
- DOI: 10.3389/fimmu.2024.1275933
T follicular helper cells expansion in transplant recipients correlates with graft infiltration and adverse outcomes
Abstract
Introduction: The process of immunization following vaccination in humans bears similarities to that of immunization with allografts. Whereas vaccination aims to elicit a rapid response, in the transplant recipient, immunosuppressants slow the immunization to alloantigens. The induction of CD4+CXCR5+ T follicular helper (Tfh) cells has been shown to correlate with the success of vaccine immunization.
Method: We studied a cohort of 65 transplant recipients who underwent histological evaluation concurrent with PBMC isolation and follow-up sampling to investigate the phenotypic profiles in the blood and allotissue and analyze their association with clinical events.
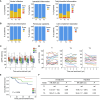
Results: The proportion of circulating Tfh cells was heterogeneous over time. Patients in whom this compartment increased had lower CCR7-PD1+CD4+CXCR5+ T cells during follow-up. These patients exhibited more alloreactive CD4+ T cells using HLA-DR-specific tetramers and a greater proportion of detectable circulating plasmablasts than the controls. Examination of baseline biopsies revealed that expansion of the circulating Tfh compartment did not follow prior intragraft leukocyte infiltration. However, multicolor immunofluorescence microscopy of the grafts showed a greater proportion of CXCR5+ T cells than in the controls. CD4+CXCR5+ cells were predominantly PD1+ and were in close contact with B cells in situ. Despite clinical stability at baseline, circulating Tfh expansion was associated with a higher risk of a composite of anti-HLA donor-specific antibodies, rejection, lower graft function, or graft loss.
Conclusion: In otherwise stable patients post-transplant, circulating Tfh expansion can identify ongoing alloreactivity, detectable before allograft injury. Tfh expansion is relevant clinically because it predicts poor graft prognosis. These findings have implications for immune surveillance.
Keywords: anti-HLA antibodies; follicular helper T cells; graft outcomes; kidney transplantation; rejection.
Copyright © 2024 Désy, Béland, Thivierge, Marcoux, Desgagnés, Bouchard-Boivin, Gama, Riopel, Latulippe and De Serres.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
T cell depletion increases humoral response by favoring T follicular helper cells expansion.Am J Transplant. 2022 Jul;22(7):1766-1778. doi: 10.1111/ajt.17038. Epub 2022 Apr 15. Am J Transplant. 2022. PMID: 35320600 Free PMC article.
-
Circulating T Follicular Helper Cell Abnormalities Associated to Different Clinical Forms of Chronic Chagas Disease.Front Cell Infect Microbiol. 2020 Mar 31;10:126. doi: 10.3389/fcimb.2020.00126. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 32296649 Free PMC article.
-
Characterization of follicular T helper cells and donor-specific T helper cells in renal transplant patients with de novo donor-specific HLA-antibodies.Clin Immunol. 2021 May;226:108698. doi: 10.1016/j.clim.2021.108698. Epub 2021 Feb 24. Clin Immunol. 2021. PMID: 33639275
-
The role of circulating T follicular helper cells in kidney transplantation.Transpl Immunol. 2021 Dec;69:101459. doi: 10.1016/j.trim.2021.101459. Epub 2021 Aug 27. Transpl Immunol. 2021. PMID: 34461243 Review.
-
Advances in T follicular helper and T follicular regulatory cells in transplantation immunity.Transplant Rev (Orlando). 2018 Oct;32(4):187-193. doi: 10.1016/j.trre.2018.07.002. Epub 2018 Jul 29. Transplant Rev (Orlando). 2018. PMID: 30139705 Review.
Cited by
-
TIGIT+Tfh show poor B-helper function and negatively correlate with SARS-CoV-2 antibody titre.Front Immunol. 2024 May 29;15:1395684. doi: 10.3389/fimmu.2024.1395684. eCollection 2024. Front Immunol. 2024. PMID: 38868776 Free PMC article.
References
-
- Schinstock CA, Mannon RB, Budde K, Chong AS, Haas M, Knechtle S, et al. . Recommended treatment for antibody-mediated rejection after kidney transplantation: the 2019 expert consensus from the transplantion society working group. Transplantation (2020) 104(5):911–22. doi: 10.1097/TP.0000000000003095 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

