Myeloid and dendritic cells enhance therapeutics-induced cytokine release syndrome features in humanized BRGSF-HIS preclinical model
- PMID: 38384461
- PMCID: PMC10880010
- DOI: 10.3389/fimmu.2024.1357716
Myeloid and dendritic cells enhance therapeutics-induced cytokine release syndrome features in humanized BRGSF-HIS preclinical model
Abstract
Objectives: Despite their efficacy, some immunotherapies have been shown to induce immune-related adverse events, including the potentially life-threatening cytokine release syndrome (CRS), calling for reliable and translational preclinical models to predict potential safety issues and investigate their rescue. Here, we tested the reliability of humanized BRGSF mice for the assessment of therapeutics-induced CRS features in preclinical settings.
Methods: BRGSF mice reconstituted with human umbilical cord blood CD34+ cells (BRGSF-CBC) were injected with anti-CD3 antibody (OKT3), anti-CD3/CD19 bispecific T-cell engager Blinatumomab, or VISTA-targeting antibody. Human myeloid and dendritic cells' contribution was investigated in hFlt3L-boosted BRGSF-CBC mice. OKT3 treatment was also tested in human PBMC-reconstituted BRGSF mice (BRGSF-PBMC). Cytokine release, immune cell distribution, and clinical signs were followed.
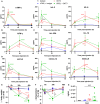
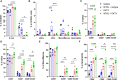
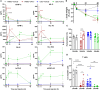
Results: OKT3 injection in BRGSF-CBC mice induced hallmark features of CRS, specifically inflammatory cytokines release, modifications of immune cell distribution and activation, body weight loss, and temperature drop. hFlt3L-boosted BRGSF-CBC mice displayed enhanced CRS features, revealing a significant role of myeloid and dendritic cells in this process. Clinical CRS-managing treatment Infliximab efficiently attenuated OKT3-induced toxicity. Comparison of OKT3 treatment's effect on BRGSF-CBC and BRGSF-PBMC mice showed broadened CRS features in BRGSF-CBC mice. CRS-associated features were also observed in hFlt3L-boosted BRGSF-CBC mice upon treatment with other T-cell or myeloid-targeting compounds.
Conclusions: These data show that BRGSF-CBC mice represent a relevant model for the preclinical assessment of CRS and CRS-managing therapies. They also confirm a significant role of myeloid and dendritic cells in CRS development and exhibit the versatility of this model for therapeutics-induced safety assessment.
Keywords: BRGSF mice; cytokine release syndrome; humanized preclinical models; immunotherapy; myeloid cells; safety assessment.
Copyright © 2024 Martin, Gonon, Martin-Jeantet, Renart-Depontieu, Biesova, Cifuentes, Mukherjee, Thisted, Doerner, Campbell, Bourré, van der Horst, Rezza and Thiam.
Conflict of interest statement
Authors GM, AG, PM-J, FR-D, AR, and KT were employed by the company genOway. Authors ZB, AC, AM, TT, and EH were employed by the company Sensei Biotherapeutics. Authors AD, DC, and LB were employed by the company Crown Bioscience. The BRGSF strain is commercialized by genOway. genOway also provides commercial immuno-oncology services. The authors declare that this study received funding from genOway and Sensei Biotherapeutics. The funders had the following involvement in the study: study design, data collection and analysis, decision to publish and preparation of the manuscript.
Figures

References
-
- Abramowicz D, Schandene L, Goldman M, Crusiaux A, Vereerstraeten P, De Pauw L, et al. . Release of tumor necrosis factor, interleukin-2, and gamma-interferon in serum after injection of OKT3 monoclonal antibody in kidney transplant recipients. Transplantation (1989) 47(4):606–8. doi: 10.1097/00007890-198904000-00008 - DOI - PubMed
-
- Winkler U, Jensen M, Manzke O, Schulz H, Diehl V, Engert A. Cytokine-release syndrome in patients with B-cell chronic lymphocytic leukemia and high lymphocyte counts after treatment with an anti-CD20 monoclonal antibody (rituximab, IDEC-C2B8). Blood (1999) 94(7):2217–24. doi: 10.1182/blood.V94.7.2217.419k02_2217_2224 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

