Tofacitinib in Patients Hospitalized With Moderate and Severe COVID-19: Not Just Another Kinase Inhibitor
- PMID: 38384612
- PMCID: PMC10880578
- DOI: 10.7759/cureus.52725
Tofacitinib in Patients Hospitalized With Moderate and Severe COVID-19: Not Just Another Kinase Inhibitor
Abstract
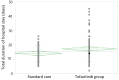
Background There has been an intense search for pharmacological agents that can complement corticosteroid therapy in the treatment of severe coronavirus disease 2019 (COVID-19). The Janus kinase inhibitor tofacitinib has shown promise in this regard. This study aimed to determine the impact of adding tofacitinib to standard care on the mortality and total duration of hospital stay in severe COVID-19. Methodology This retrospective study compared the mortality and total duration of hospital stay among patients admitted with severe COVID-19 to a designated COVID-19 hospital in south India who had received tofacitinib in addition to standard care versus standard care alone. Medical case records of severe COVID-19 patients were retrieved and screened for inclusion. Categorical variables such as mortality were expressed as proportions and compared using the chi-square test, while continuous variables such as total duration of hospital stay were compared via the independent t-test. The odds ratio (OR) was calculated for the mortality difference between the two groups. P-values ≤0.05 were considered significant. Results Following the initial screening of 250 medical records, 186 patients were included in the final analysis, of whom 103 had received tofacitinib and 83 had received standard care. There was no significant difference in mortality between the two groups (OR = 1.58 (95% confidence interval = 0.71 to 3.51); p = 0.26). The total duration of hospital stay was significantly longer among those in the tofacitinib group (17.14 ± 8.85 days vs. 14.04 ± 5.48 days; p = 0.01). Conclusions Tofacitinib did not improve the clinical outcomes when used to supplement corticosteroids in the treatment of severe COVID-19.
Keywords: corticosteroids; covid-19; cytokines; janus kinase inhibitors; tofacitinib.
Copyright © 2024, Shankar et al.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- COVID COVID, W. (19. WHO COVID-19 dashboard. [ May; 2022 ]. 2020. https://data.who.int/dashboards/covid19/cases?n=c https://data.who.int/dashboards/covid19/cases?n=c
LinkOut - more resources
Full Text Sources