Effects of non-invasive vagus nerve stimulation on clinical symptoms and molecular biomarkers in Parkinson's disease
- PMID: 38384731
- PMCID: PMC10879328
- DOI: 10.3389/fnagi.2023.1331575
Effects of non-invasive vagus nerve stimulation on clinical symptoms and molecular biomarkers in Parkinson's disease
Abstract
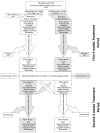
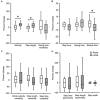
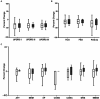
Non-invasive vagus nerve stimulation (nVNS) is an established neurostimulation therapy used in the treatment of epilepsy, migraine and cluster headache. In this randomized, double-blind, sham-controlled trial we explored the role of nVNS in the treatment of gait and other motor symptoms in Parkinson's disease (PD) patients. In a subgroup of patients, we measured selected neurotrophins, inflammatory markers and markers of oxidative stress in serum. Thirty-three PD patients with freezing of gait (FOG) were randomized to either active nVNS or sham nVNS. After baseline assessments, patients were instructed to deliver six 2 min stimulations (12 min/day) of the active nVNS/sham nVNS device for 1 month at home. Patients were then re-assessed. After a one-month washout period, they were allocated to the alternate treatment arm and the same process was followed. Significant improvements in key gait parameters (speed, stance time and step length) were observed with active nVNS. While serum tumor necrosis factor- α decreased, glutathione and brain-derived neurotrophic factor levels increased significantly (p < 0.05) after active nVNS treatment. Here we present the first evidence of the efficacy and safety of nVNS in the treatment of gait in PD patients, and propose that nVNS can be used as an adjunctive therapy in the management of PD patients, especially those suffering from FOG. Clinical trial registration: identifier ISRCTN14797144.
Keywords: Parkinson’s disease; gait; neuroinflammation; oxidative stress; vagus nerve stimulation.
Copyright © 2024 Mondal, Choudhury, Banerjee, Roy, Chatterjee, Basu, Singh, Halder, Shubham, Baker, Baker and Kumar.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Non-invasive vagus nerve stimulation improves clinical and molecular biomarkers of Parkinson's disease in patients with freezing of gait.NPJ Parkinsons Dis. 2021 May 27;7(1):46. doi: 10.1038/s41531-021-00190-x. NPJ Parkinsons Dis. 2021. Retraction in: NPJ Parkinsons Dis. 2022 Nov 3;8(1):148. doi: 10.1038/s41531-022-00424-6. PMID: 34045464 Free PMC article. Retracted.
-
Safety and tolerability of adjunct non-invasive vagus nerve stimulation in people with parkinson's: a study protocol.BMC Neurol. 2023 Feb 3;23(1):58. doi: 10.1186/s12883-023-03081-1. BMC Neurol. 2023. PMID: 36737716 Free PMC article.
-
Inter-ictal assay of peripheral circulating inflammatory mediators in migraine patients under adjunctive cervical non-invasive vagus nerve stimulation (nVNS): A proof-of-concept study.Brain Stimul. 2019 May-Jun;12(3):643-651. doi: 10.1016/j.brs.2019.01.008. Epub 2019 Jan 19. Brain Stimul. 2019. PMID: 30745260 Clinical Trial.
-
Effectiveness of non-invasive vagal nerve stimulation in Parkinson's disease: A comprehensive systematic review and meta-analysis.J Clin Neurosci. 2025 Mar;133:111016. doi: 10.1016/j.jocn.2024.111016. Epub 2024 Dec 28. J Clin Neurosci. 2025. PMID: 39733649
-
Noninvasive vagus nerve stimulation in Parkinson's disease: current status and future prospects.Expert Rev Med Devices. 2021 Oct;18(10):971-984. doi: 10.1080/17434440.2021.1969913. Epub 2021 Sep 8. Expert Rev Med Devices. 2021. PMID: 34461787 Review.
Cited by
-
Unraveling the threads of stability: A review of the neurophysiology of postural control in Parkinson's disease.Neurotherapeutics. 2024 Apr;21(3):e00354. doi: 10.1016/j.neurot.2024.e00354. Epub 2024 Apr 4. Neurotherapeutics. 2024. PMID: 38579454 Free PMC article. Review.
-
Transcutaneous vagus nerve stimulation for Parkinson's disease: a systematic review and meta-analysis.Front Aging Neurosci. 2025 Jan 14;16:1498176. doi: 10.3389/fnagi.2024.1498176. eCollection 2024. Front Aging Neurosci. 2025. PMID: 39877075 Free PMC article.
-
Impact of Stimulation Duration in taVNS-Exploring Multiple Physiological and Cognitive Outcomes.Brain Sci. 2024 Aug 29;14(9):875. doi: 10.3390/brainsci14090875. Brain Sci. 2024. PMID: 39335371 Free PMC article.
-
Transcutaneous auricular vagus nerve stimulation improves cortical functional topological properties and intracortical facilitation in patients with Parkinson's disease.NPJ Parkinsons Dis. 2025 Mar 1;11(1):38. doi: 10.1038/s41531-025-00889-1. NPJ Parkinsons Dis. 2025. PMID: 40025047 Free PMC article.
References
-
- Benjamini Y., Hochberg Y. (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc., Ser. B, Methodol. 57, 289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x - DOI
LinkOut - more resources
Full Text Sources

