Airway inflammation accelerates pulmonary exacerbations in cystic fibrosis
- PMID: 38384849
- PMCID: PMC10879674
- DOI: 10.1016/j.isci.2024.108835
Airway inflammation accelerates pulmonary exacerbations in cystic fibrosis
Abstract
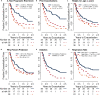
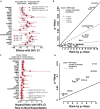
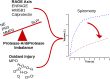
Airway inflammation underlies cystic fibrosis (CF) pulmonary exacerbations. In a prospective multicenter study of randomly selected, clinically stable adolescents and adults, we assessed relationships between 24 inflammation-associated molecules and the future occurrence of CF pulmonary exacerbation using proportional hazards models. We explored relationships for potential confounding or mediation by clinical factors and assessed sensitivities to treatments including CF transmembrane regulator (CFTR) protein synthesis modulators. Results from 114 participants, including seven on ivacaftor or lumacaftor-ivacaftor, representative of the US CF population during the study period, identified 10 biomarkers associated with future exacerbations mediated by percent predicted forced expiratory volume in 1 s. The findings were not sensitive to anti-inflammatory, antibiotic, and CFTR modulator treatments. The analyses suggest that combination treatments addressing RAGE-axis inflammation, protease-mediated injury, and oxidative stress might prevent pulmonary exacerbations. Our work may apply to other airway inflammatory diseases such as bronchiectasis and the acute respiratory distress syndrome.
Keywords: Health sciences; Medical specialty; Medicine; Omics; respiratory medicine.
© 2024 The Author(s).
Conflict of interest statement
T.G.L., J.A.F., J.L.J., Y.L., K.A.P., and J.B.V. received other support from the CFF (CC132-16AD, LIOU14Y0, LIOU14P0) and the National Heart Lung and Blood Institute (NHLBI) of the National Institutes of Health (NIH) (R01 HL125520) and received support during the current study for performing clinical trials from Abbvie, Calithera Biosciences, Corbus Pharmaceuticals, Gilead Sciences, Laurent Pharmaceuticals, Nivalis Therapeutics, Novartis, Proteostasis, Savara Pharmaceuticals, Translate Bio, and Vertex Pharmaceuticals. F.R.A. received additional other support from the NHLBI/NIH (R01 HL125520), the National Science Foundation (EMSW21-RTG), and the Margolis Family Foundation of Utah. P.S.B. received other support from the CFF (Center and TDC grants) and the NHLBI/NIH (U01 HL114623) and received support for a clinical trial from Alcresta Therapeutics. B.A.C. received other support from the CFF (C112–12, C112-TDC09Y, 10063SUB, 41339154.s132P010379SUB) and received support for clinical trials from Genentech, Novartis, and Vertex Pharmaceuticals. C.L.D. received other support from the CFF (C004–11, C004-TDC09Y, DAINES11Y3) and from the Health Resources and Services Administration (T72MC00012). J.A.F. transitioned to become an employee of ICON plc, a clinical research organization involved in various trials pertinent to CF during the study and is now an employee of Vertex Pharmaceuticals; JAF, ICON, and Vertex had no direct involvement in performance of the study following the initial change in affiliation. T.H. received other support from the CFF (PACE, Center Grant) and received support for clinical trials from Celtaxsys and Vertex Pharmaceuticals. J.R.H. received other support from the NHLBI/NIH (HHSN268200900018C) and the Veterans Administration Healthcare System (I01 BX001533). J.L. received other support from the CFF (C017-11AF). C.N. received other support from the CFF (C138-12). P.R. received other support from the CFF (C003–12, C003-TDC09Y). S.D.S. received other support from the CFF (AQUADEK12K1, SAGEL11CS0, GOAL13K2, NICK13A0, SAGEL14K1, NICK15R0) and the NHLBI/NIH (U54 HL096458) and the NCATS/NIH (Colorado CTSA Grant Number UL1 TR002535). J.L.T.-C. received other support from the CFF (TDC) and the NHLBI/NIH (HL103801) and received support for clinical trials from Vertex Pharmaceuticals. K.N.O. was funded by the intramural research program of the NHLBI, NIH, during the study. Neither the project sponsors nor any sources of other support had direct roles in development and conduct of the study. None of the sponsors of clinical trials mentioned earlier participated in any way with this trial.
Figures



References
-
- Flume P.A., Wainwright C.E., Elizabeth Tullis D., Rodriguez S., Niknian M., Higgins M., Davies J.C., Wagener J.S. Recovery of lung function following a pulmonary exacerbation in patients with cystic fibrosis and the G551D-CFTR mutation treated with ivacaftor. J. Cyst. Fibros. 2018;17:83–88. doi: 10.1016/j.jcf.2017.06.002. - DOI - PubMed
-
- Casey M., Gabillard-Lefort C., McElvaney O.F., McElvaney O.J., Carroll T., Heeney R.C., Gunaratnam C., Reeves E.P., Murphy M.P., McElvaney N.G. Effect of elexacaftor/tezacaftor/ivacaftor on airway and systemic inflammation in cystic fibrosis. Thorax. 2023;78:835–839. doi: 10.1136/thorax-2022-219943. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

