Imaging spectrum of spinal dysraphism: A diagnostic challenge
- PMID: 38384981
- PMCID: PMC10879466
- DOI: 10.4102/sajr.v27i1.2747
Imaging spectrum of spinal dysraphism: A diagnostic challenge
Abstract
Spinal dysraphism (SD) is a collective term for congenital malformations of the spine and spinal cord. It includes a wide range of congenital anomalies resulting from aberrations in the stages of gastrulation, primary neurulation and secondary neurulation. Spinal dysraphism may lead to neurological impairment of varying severity including weakness of the extremities, incontinence of bowel and bladder, sexual dysfunction, among others. Diagnosis of SDs is quite challenging because of its wide spectrum and complex cascade of embryologic events. Knowledge of normal embryology and proper understanding of imaging features of SD are important for early accurate diagnosis.
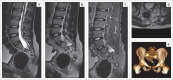
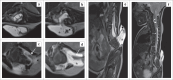
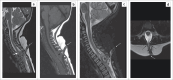
Contribution: This series of five cases describes the imaging spectrum of spinal dysraphism and highlights the embryological basis for their development, which could facilitate early correct diagnosis, surgical planning and reduced morbidity related to these malformations. It also includes an extremely rare case of complex spinal dysraphism (Type II diastematomyelia with right hemimyelomeningocoele and left hemilipomyelomeningocoele) with Chiari II malformation.
Keywords: caudal regression syndrome; diastematomyelia; dorsal dermal sinus; hemimyelomeningocoele; lipomyelocoele; lipomyelomeningocoele; myelomeningocoele; spinal dysraphism.
© 2023. The Authors.
Conflict of interest statement
The authors declare that they have no personal or financial relationship that may have inappropriately influenced the writing of this article.
Figures



References
-
- Jans L, Vlummens P, Van Damme S, Verstraete K, Abernethy L. Hemimyelomeningocele: A rare and complex spinal dysraphism. JBR-BTR. 2008;91(5):198–199. - PubMed
LinkOut - more resources
Full Text Sources
