Development of ovalbumin implants with different spatial configurations for treatment of peripheral nerve injury
- PMID: 38384987
- PMCID: PMC10879707
- DOI: 10.1016/j.bioactmat.2024.01.025
Development of ovalbumin implants with different spatial configurations for treatment of peripheral nerve injury
Abstract
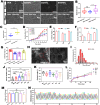
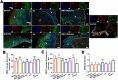
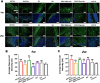
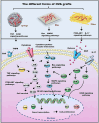
Peripheral nerve injury (PNI) seriously affects the health and life of patients, and is an urgent clinical problem that needs to be resolved. Nerve implants prepared from various biomaterials have played a positive role in PNI, but the effect should be further improved and thus new biomaterials is urgently needed. Ovalbumin (OVA) contains a variety of bioactive components, low immunogenicity, tolerance, antimicrobial activity, non-toxicity and biodegradability, and has the ability to promote wound healing, cell growth and antimicrobial properties. However, there are few studies on the application of OVA in neural tissue engineering. In this study, OVA implants with different spatial structures (membrane, fiber, and lyophilized scaffolds) were constructed by casting, electrospinning, and freeze-drying methods, respectively. The results showed that the OVA implants had excellent physicochemical properties and were biocompatible without significant toxicity, and can promote vascularization, show good histocompatibility, without excessive inflammatory response and immunogenicity. The in vitro results showed that OVA implants could promote the proliferation and migration of Schwann cells, while the in vivo results confirmed that OVA implants (the E5/70% and 20 kV 20 μL/min groups) could effectively regulate the growth of blood vessels, reduce the inflammatory response and promote the repair of subcutaneous nerve injury. Further on, the high-throughput sequencing results showed that the OVA implants up-regulated differential expression of genes related to biological processes such as tumor necrosis factor-α (TNF-α), phosphatidylinositide 3-kinases/protein kinase B (PI3K-Akt) signaling pathway, axon guidance, cellular adhesion junctions, and nerve regeneration in Schwann cells. The present study is expected to provide new design concepts and theoretical accumulation for the development of a new generation of nerve regeneration implantable biomaterials.
Keywords: Casting method; Electrospinning; Freeze drying; Nerve scaffold; Ovalbumin; Peripheral nerve injury.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures







Similar articles
-
Surface topologized ovalbumin scaffolds containing YIGSR peptides for modulating Schwann cell behavior.Int J Biol Macromol. 2023 Dec 31;253(Pt 4):127015. doi: 10.1016/j.ijbiomac.2023.127015. Epub 2023 Sep 25. Int J Biol Macromol. 2023. PMID: 37758111
-
Down-regulation miR-146a-5p in Schwann cell-derived exosomes induced macrophage M1 polarization by impairing the inhibition on TRAF6/NF-κB pathway after peripheral nerve injury.Exp Neurol. 2023 Apr;362:114295. doi: 10.1016/j.expneurol.2022.114295. Epub 2022 Dec 6. Exp Neurol. 2023. PMID: 36493861
-
A compound scaffold with uniform longitudinally oriented guidance cues and a porous sheath promotes peripheral nerve regeneration in vivo.Acta Biomater. 2018 Mar 1;68:223-236. doi: 10.1016/j.actbio.2017.12.010. Epub 2017 Dec 20. Acta Biomater. 2018. PMID: 29274478
-
Advances in novel biomaterials combined with traditional Chinese medicine rehabilitation technology in treatment of peripheral nerve injury.Front Neurol. 2024 Jun 13;15:1421772. doi: 10.3389/fneur.2024.1421772. eCollection 2024. Front Neurol. 2024. PMID: 38938781 Free PMC article. Review.
-
Biomechanical microenvironment in peripheral nerve regeneration: from pathophysiological understanding to tissue engineering development.Theranostics. 2022 Jun 27;12(11):4993-5014. doi: 10.7150/thno.74571. eCollection 2022. Theranostics. 2022. PMID: 35836812 Free PMC article. Review.
Cited by
-
NPD1 Relieves Neuropathic Pain and Accelerates the Recovery of Motor Function After Peripheral Nerve Injury.Pain Res Manag. 2024 Oct 30;2024:1109287. doi: 10.1155/2024/1109287. eCollection 2024. Pain Res Manag. 2024. PMID: 39512892 Free PMC article.
-
Potentially commercializable nerve guidance conduits for peripheral nerve injury: Past, present, and future.Mater Today Bio. 2025 Feb 5;31:101503. doi: 10.1016/j.mtbio.2025.101503. eCollection 2025 Apr. Mater Today Bio. 2025. PMID: 40018056 Free PMC article. Review.
-
An ovalbumin-based hydrogel loaded with dendrobium polysaccharide for promoting wound healing while reducing inflammations.Sci Rep. 2025 Jul 1;15(1):21825. doi: 10.1038/s41598-025-06717-z. Sci Rep. 2025. PMID: 40595006 Free PMC article.
References
-
- Qian Y., Lin H., Yan Z., Shi J., Fan C. Functional nanomaterials in peripheral nerve regeneration: scaffold design, chemical principles and microenvironmental remodeling. Mater. Today. 2021;51:165–187. doi: 10.1016/j.mattod.2021.09.014. - DOI
-
- Zheng T., Wu L., Sun S., Xu J., Han Q., Liu Y., Wu R., Li G. Co-culture of Schwann cells and endothelial cells for synergistically regulating dorsal root ganglion behavior on chitosan-based anisotropic topology for peripheral nerve regeneration. Burns Trauma. 2022;10 doi: 10.1093/burnst/tkac030. tkac030. - DOI - PMC - PubMed
-
- Gao S., Chen X., Lu B., Meng K., Zhang K.Q., Zhao H. Recent advances on nerve guide conduits based on textile methods. Smart Mater Med. 2023;4:368–383. doi: 10.1016/j.smaim.2022.12.001. - DOI
LinkOut - more resources
Full Text Sources

