Analysis of differentially expressed proteins in lymph fluids related to lymphatic metastasis in a breast cancer rabbit model guided by contrast‑enhanced ultrasound
- PMID: 38385114
- PMCID: PMC10879953
- DOI: 10.3892/ol.2024.14276
Analysis of differentially expressed proteins in lymph fluids related to lymphatic metastasis in a breast cancer rabbit model guided by contrast‑enhanced ultrasound
Abstract
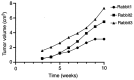
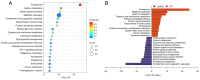
The aim of the present study was to identify differentially expressed proteins in the lymph fluid of rabbits with breast cancer lymphatic metastasis compared with healthy rabbits and to analyze and verify these proteins using proteomics technologies. In the process of breast cancer metastasis, the composition of the lymph fluid will also change. Rabbits with breast cancer lymph node metastasis and normal rabbits were selected for analysis. Lymph fluid was extracted under the guidance of percutaneous contrast-enhanced ultrasound. Label-free quantitative proteomics was used to detect and compare differences between the rabbit cancer model and healthy rabbits and differential protein expression results were obtained. Bioinformatics analysis was performed using Kyoto Encyclopedia of Genes and Genomes and Gene Ontology analysis software, selecting the most significantly differentially expressed proteins. Finally, parallel reaction monitoring technology was applied for validation. A total of 547 significantly differentially expressed proteins were found in the present study, which included 371 upregulated proteins and 176 downregulated proteins. The aforementioned genes were mainly involved in various cellular and metabolic pathways, including upregulated proteins, such as biliverdin reductase A and isocitrate dehydrogenase 2 and downregulated proteins, such as pyridoxal kinase. The upregulated proteins protein disulfide-isomerase 3, protein kinase cAMP-dependent type I regulatory subunit α and ATP-binding cassette sub-family C member 4 participated in immune regulation, endocrine regulation and anti-tumor drug resistance regulation, respectively. Compared with healthy rabbits, rabbits with breast cancer metastasis differentially expressed of a number of different proteins in their lymph, which participate in the pathophysiological process of tumor occurrence and metastasis. Through further research, these differential proteins can be used as predictive indicators of breast cancer metastasis and new therapeutic targets.
Keywords: breast cancer; contrast-enhanced ultrasonography; differentially expressed protein; lymph node metastasis; proteomics.
Copyright: © Xu et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




Similar articles
-
Study on the Relationship Between Differentially Expressed Proteins in Breast Cancer and Lymph Node Metastasis.Adv Ther. 2023 Sep;40(9):4004-4023. doi: 10.1007/s12325-023-02588-w. Epub 2023 Jul 9. Adv Ther. 2023. PMID: 37422893
-
[Identification of the differentially expressed genes between primary breast cancer and paired lymph node metastasis through combining mRNA differential display and gene microarray].Zhonghua Yi Xue Za Zhi. 2006 Oct 24;86(39):2749-55. Zhonghua Yi Xue Za Zhi. 2006. PMID: 17199993 Chinese.
-
Genes whose expressions in the primary lung squamous cell carcinoma are able to accurately predict the progression of metastasis through lymphatic system, inferred from a bioinformatics analyses.Sci Rep. 2023 Apr 25;13(1):6733. doi: 10.1038/s41598-023-33897-3. Sci Rep. 2023. PMID: 37185598 Free PMC article.
-
Identification of novel hub genes associated with lymph node metastasis of head and neck squamous cell carcinoma by completive bioinformatics analysis.Ann Transl Med. 2021 Nov;9(22):1678. doi: 10.21037/atm-21-5704. Ann Transl Med. 2021. PMID: 34988187 Free PMC article.
-
Prognostic value of biomarkers EpCAM and αB-crystallin associated with lymphatic metastasis in breast cancer by iTRAQ analysis.BMC Cancer. 2019 Aug 23;19(1):831. doi: 10.1186/s12885-019-6016-3. BMC Cancer. 2019. PMID: 31443698 Free PMC article.
Cited by
-
Sonication-assisted protein extraction improves proteomic detection of membrane-bound and DNA-binding proteins from tumor tissues.Nat Protoc. 2025 Aug;20(8):2083-2099. doi: 10.1038/s41596-024-01113-9. Epub 2025 Feb 17. Nat Protoc. 2025. PMID: 39962197 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
