A NOTCH3-CXCL12-driven myeloma-tumor niche signaling axis promotes chemoresistance in multiple myeloma
- PMID: 38385272
- PMCID: PMC11290536
- DOI: 10.3324/haematol.2023.284443
A NOTCH3-CXCL12-driven myeloma-tumor niche signaling axis promotes chemoresistance in multiple myeloma
Abstract
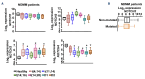
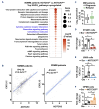
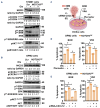
Multiple myeloma (MM) remains incurable due to disease relapse and drug resistance. Notch signals from the tumor microenvironment (TME) confer chemoresistance, but the cellular and molecular mechanisms are not entirely understood. Using clinical and transcriptomic datasets, we found that NOTCH3 is upregulated in CD138+ cells from newly diagnosed MM (NDMM) patients compared to healthy individuals and increased in progression/relapsed MM (PRMM) patients. Further, NDMM patients with high NOTCH3 expression exhibited worse responses to bortezomib (BOR)-based therapies. Cells of the TME, including osteocytes, upregulated NOTCH3 in MM cells and protected them from apoptosis induced by BOR. NOTCH3 activation (NOTCH3OE) in MM cells decreased BOR anti-MM efficacy and its ability to improve survival in in vivo myeloma models. Molecular analyses revealed that NDMM and PRMM patients with high NOTCH3 exhibit CXCL12 upregulation. TME cells upregulated CXCL12 and activated the CXCR4 pathway in MM cells in a NOTCH3-dependent manner. Moreover, genetic or pharmacologic inhibition of CXCL12 in NOTCH3OE MM cells restored sensitivity to BOR regimes in vitro and in human bones bearing NOTCH3OE MM tumors cultured ex vivo. Our clinical and preclinical data unravel a novel NOTCH3-CXCL12 pro-survival signaling axis in the TME and suggest that osteocytes transmit chemoresistance signals to MM cells.
Figures




References
-
- Nefedova Y, Landowski TH, Dalton WS. Bone marrow stromal-derived soluble factors and direct cell contact contribute to de novo drug resistance of myeloma cells by distinct mechanisms. Leukemia. 2003;17(6):1175-1182. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

