Selective pharmacologic targeting of CTPS1 shows single-agent activity and synergizes with BCL2 inhibition in aggressive mantle cell lymphoma
- PMID: 38385294
- PMCID: PMC11290505
- DOI: 10.3324/haematol.2023.284345
Selective pharmacologic targeting of CTPS1 shows single-agent activity and synergizes with BCL2 inhibition in aggressive mantle cell lymphoma
Abstract
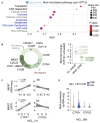
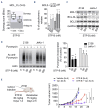
Innovative therapeutic strategies have emerged over the past decade to improve outcomes for most lymphoma patients. Nevertheless, the aggressive presentation seen in high-risk mantle cell lymphoma (MCL) patients remains an unmet medical need. The highly proliferative cells that characterize these tumors depend on nucleotide synthesis to ensure high DNA replication and RNA synthesis. To take advantage of this vulnerability, STP-B, a clinically available small molecule selectively targeting CTP synthase 1 (CTPS1) has been recently developed. CTPS1 is a key enzyme of the pyrimidine synthesis pathway mediated through its unique ability to provide enough CTP in highly proliferating cells. Herein, we demonstrated that CTPS1 was expressed in all MCL cells, and that its high expression was associated with unfavorable outcomes for patients treated with chemotherapy. Using aggressive MCL models characterized by blastoid morphology, TP53 mutation or polyresistance to targeted therapies, we showed that STP-B was highly effective at nanomolar concentrations in vitro and in vivo, irrespective of these high-risk features. Inhibition of CTPS1 rapidly leads to cell cycle arrest in early S-phase accompanied by inhibition of translation, including of the anti-apoptotic protein MCL1. Consequently, CTPS1 inhibition induced synergistic cell death in combination with the selective BCL2 inhibitor venetoclax, both in vitro and in vivo. Overall, our study identified CTPS1 as a promising target for MCL patients and provided a mechanism-based combination with the BCL2 inhibitor venetoclax for the design of future chemotherapy-free treatment regimens to overcome resistance.
Figures



References
-
- Wang ML, Jurczak W, Jerkeman M, et al. . Ibrutinib plus bendamustine and rituximab in untreated mantle-cell lymphoma. N Engl J Med. 2022;386(26):2482-2494. - PubMed
-
- Dreyling M, Doorduijn JK, Gine E, et al. . Efficacy and safety of ibrutinib combined with standard first-line treatment or as substitute for autologous stem cell transplantation in younger patients with mantle cell lymphoma: results from the randomized triangle trial by the european MCL network. Blood. 2022;140(Suppl 1):1-3. - PubMed
-
- Le Gouill S, Morschhauser F, Chiron D, et al. . Ibrutinib, obinutuzumab, and venetoclax in relapsed and untreated patients with mantle cell lymphoma: a phase 1/2 trial. Blood. 2021;137(7):877-887. - PubMed
-
- Eichhorst B, Niemann CU, Kater AP, et al. . First-line venetoclax combinations in chronic lymphocytic leukemia. N Engl J Med. 2023;388(19):1739-1754. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

