Long-term outcomes of the pentaspline pulsed-field ablation catheter for the treatment of paroxysmal atrial fibrillation: results of the prospective, multicentre FARA-Freedom Study
- PMID: 38385529
- PMCID: PMC10932745
- DOI: 10.1093/europace/euae053
Long-term outcomes of the pentaspline pulsed-field ablation catheter for the treatment of paroxysmal atrial fibrillation: results of the prospective, multicentre FARA-Freedom Study
Abstract
Aims: Pulmonary vein isolation (PVI) is a well-established strategy for the treatment of paroxysmal atrial fibrillation (PAF). Despite randomized controlled trials and real-world data showing the promise of pulsed-field ablation (PFA) for this treatment, long-term efficacy and safety data demonstrating single-procedure outcomes off antiarrhythmic drugs remain limited. The aim of the FARA-Freedom Study was to evaluate the long-term efficacy and safety of PFA using the pentaspline catheter for PAF.
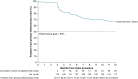
Methods and results: FARA-Freedom, a prospective, non-randomized, multicentre study, enrolled patients with PAF undergoing de novo PVI with PFA, who were followed for 12 months with weekly transtelephonic monitoring and a 72-h Holter ECG at 6 and 12 months. The primary safety endpoint was a composite of device- or procedure-related serious adverse events out to 7 days post-ablation and PV stenosis or atrioesophageal (AE) fistula out to 12 months. Treatment success is a composite of acute PVI and chronic success, which includes freedom from any documented atrial tachyarrhythmia longer than 30 s, use of antiarrhythmic drugs or cardioversion after a 3-month blanking period, or use of amiodarone or repeat ablation at any time. The study enrolled 179 PAF patients (62 ± 10 years, 39% female) at 13 centres. At the index procedure, all PVs were successfully isolated with the pentaspline PFA catheter. Procedure and left atrial dwell times, with a 20-min waiting period, were 71.9 ± 17.6 and 41.0 ± 13.3 min, respectively. Fluoroscopy time was 11.5 ± 7.4 min. Notably, monitoring compliance was high, with 88.4 and 90.3% with weekly events and 72-h Holter monitors, respectively. Freedom from the composite primary effectiveness endpoint was 66.6%, and 41 patients had atrial tachyarrhythmia recurrence, mostly recurrent atrial fibrillation (31 patients). The composite safety endpoint occurred in two patients (1.1%), one tamponade and one transient ischaemic attack. There was no coronary spasm, PV stenosis, or AE fistula. There were four cases of transient phrenic nerve palsy, but all resolved during the index procedure.
Conclusion: In this prospective, non-randomized, multicentre study, PVI using a pentaspline PFA catheter was effective in treating PAF patients despite rigourous endpoint definitions and high monitoring compliance and demonstrated favourable safety.
Registration: Clinical Trials.gov Identifier: NCT05072964 (sponsor: Boston Scientific Corporation).
Keywords: Atrial fibrillation; Paroxysmal atrial fibrillation; Pentaspline PFA catheter; Pulsed field ablation.
© The Author(s) 2024. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest: We have read the journal’s policy, and the authors of this manuscript have the following competing interests: Authors have served as consultants and received lecture honoraria or research grants from multiple industry partners, including Boston Scientific (the trial sponsor), Biosense Webster, Medtronic, Abbott, Lifetech, Bayer, and Bristol Myers Squibb. Additionally, the following are employees of the sponsor: A.V., A.B.A., M.J., and J.D.R.
Figures
References
-
- Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 2021;42:373–498. - PubMed
-
- Reddy VY, Gerstenfeld EP, Natale A, Whang W, Cuoco FA, Patel C et al. Pulsed field or conventional thermal ablation for paroxysmal atrial fibrillation. N Engl J Med 2023;389:1660–71. - PubMed
-
- Reddy VY, Dukkipati SR, Neuzil P, Anic A, Petru J, Funasako M et al. Pulsed field ablation of paroxysmal atrial fibrillation: 1-year outcomes of IMPULSE, PEFCAT, and PEFCAT II. JACC Clin Electrophysiol 2021;7:614–27. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials