Safety and Immunogenicity of an mRNA-Based RSV Vaccine Including a 12-Month Booster in a Phase 1 Clinical Trial in Healthy Older Adults
- PMID: 38385566
- PMCID: PMC11420773
- DOI: 10.1093/infdis/jiae081
Safety and Immunogenicity of an mRNA-Based RSV Vaccine Including a 12-Month Booster in a Phase 1 Clinical Trial in Healthy Older Adults
Abstract
Background: An mRNA-based respiratory syncytial virus (RSV) vaccine, mRNA-1345, is under clinical investigation to address RSV disease burden in older adults.
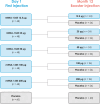
Methods: Based on a randomized, observer-blind, placebo-controlled design, this phase 1 dose-ranging study evaluated the safety, reactogenicity, and immunogenicity of mRNA-1345 in adults aged 65 to 79 years. Participants were randomized to receive 1 dose of mRNA-1345 (12.5, 25, 50, 100, or 200 µg) or placebo and matched mRNA-1345 booster or placebo at 12 months.
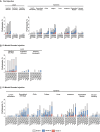
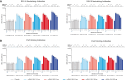
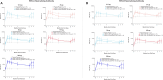
Results: Overall, 298 participants received the first injection and 247 received the 12-month booster injection. mRNA-1345 was generally well tolerated after both injections, with the most frequently reported solicited adverse reactions being injection site pain, fatigue, headache, arthralgia, and myalgia. Reactogenicity was higher after the booster injection but with severity, time to onset, and duration similar to the first injection. A single mRNA-1345 injection boosted RSV-A and RSV-B neutralizing antibody titers and prefusion F binding antibody (preF bAb) concentrations at 1 month (geometric mean fold rises: RSV-A, 10.2-16.5; RSV-B, 5.3-12.5; preF bAb, 7.2-12.1). RSV antibody levels remained above baseline through 12 months, indicating immune persistence. A 12-month booster injection also increased RSV-A and RSV-B neutralizing antibody titers and preF bAb concentrations; titers after booster injection were numerically lower than those after the first dose, with overlapping 95% CIs.
Conclusions: mRNA-1345 was well tolerated and immunogenic following a single injection and a 12-month booster.
Clinical trials registration: NCT04528719 (ClinicalTrials.gov).
Keywords: mRNA vaccine; mRNA-1345; older adult; respiratory syncytial virus; safety and immunogenicity.
© The Author(s) 2024. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. C. A. S., R. M., A. K., R. D., L. W., C. A. P., J. G., E. W., R. G., A. K. S., C. R., S. K. S., and G. L. C. are employees of Moderna, Inc, and may hold stock/stock options in the company. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures
References
-
- Li Y, Reeves RM, Wang X, et al. Global patterns in monthly activity of influenza virus, respiratory syncytial virus, parainfluenza virus, and metapneumovirus: a systematic analysis. Lancet Glob Health 2019; 7:e1031–45. - PubMed
-
- Centers for Disease Control and Prevention . Respiratory syncytial virus (RSV) symptoms and care. Available at: https://www.cdc.gov/rsv/about/symptoms.html#:∼:text=Take%20steps%20to%20.... Accessed 30 March 2022.
-
- Shi T, Denouel A, Tietjen AK, et al. Global disease burden estimates of respiratory syncytial virus–associated acute respiratory infection in older adults in 2015: a systematic review and meta-analysis. J Infect Dis 2020; 222:S577–83. - PubMed
-
- Centers for Disease Control and Prevention . RSV in older adults and adults with chronic medical conditions. Available at: https://www.cdc.gov/rsv/high-risk/older-adults.html#:∼:text=Older%20adul.... Accessed 5 April 2022.
-
- Centers for Disease Control and Prevention . RSV in infants and young children. Available at: https://www.cdc.gov/rsv/high-risk/infants-young-children.html. Accessed 4 April 2023.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

