Cellular iron depletion enhances behavioral rhythm by limiting brain Per1 expression in mice
- PMID: 38385622
- PMCID: PMC10883092
- DOI: 10.1111/cns.14592
Cellular iron depletion enhances behavioral rhythm by limiting brain Per1 expression in mice
Abstract
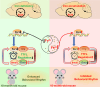
Aims: Disturbances in the circadian rhythm are positively correlated with the processes of aging and related neurodegenerative diseases, which are also associated with brain iron accumulation. However, the role of brain iron in regulating the biological rhythm is poorly understood. In this study, we investigated the impact of brain iron levels on the spontaneous locomotor activity of mice with altered brain iron levels and further explored the potential mechanisms governing these effects in vitro.
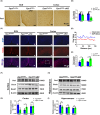
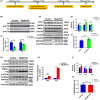
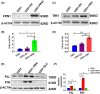
Results: Our results revealed that conditional knockout of ferroportin 1 (Fpn1) in cerebral microvascular endothelial cells led to brain iron deficiency, subsequently resulting in enhanced locomotor activity and increased expression of clock genes, including the circadian locomotor output cycles kaput protein (Clock) and brain and muscle ARNT-like 1 (Bmal1). Concomitantly, the levels of period circadian regulator 1 (PER1), which functions as a transcriptional repressor in regulating biological rhythm, were decreased. Conversely, the elevated brain iron levels in APP/PS1 mice inhibited autonomous rhythmic activity. Additionally, our findings demonstrate a significant decrease in serum melatonin levels in Fpn1cdh5 -CKO mice compared with the Fpn1flox/flox group. In contrast, APP/PS1 mice with brain iron deposition exhibited higher serum melatonin levels than the WT group. Furthermore, in the human glioma cell line, U251, we observed reduced PER1 expression upon iron limitation by deferoxamine (DFO; iron chelator) or endogenous overexpression of FPN1. When U251 cells were made iron-replete by supplementation with ferric ammonium citrate (FAC) or increased iron import through transferrin receptor 1 (TfR1) overexpression, PER1 protein levels were increased. Additionally, we obtained similar results to U251 cells in mouse cerebellar astrocytes (MA-c), where we collected cells at different time points to investigate the rhythmic expression of core clock genes and the impact of DFO or FAC treatment on PER1 protein levels.
Conclusion: These findings collectively suggest that altered iron levels influence the circadian rhythm by regulating PER1 expression and thereby modulating the molecular circadian clock. In conclusion, our study identifies the regulation of brain iron levels as a potential new target for treating age-related disruptions in the circadian rhythm.
Keywords: Per1; aging; circadian rhythm; clock genes; iron.
© 2024 The Authors. CNS Neuroscience & Therapeutics published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures



Similar articles
-
Obese Neotomodon alstoni mice exhibit sexual dimorphism in the daily profile of circulating melatonin and clock proteins PER1 and BMAL1 in the hypothalamus and peripheral oscillators.Chronobiol Int. 2021 Apr;38(4):584-597. doi: 10.1080/07420528.2020.1860999. Epub 2021 Jan 3. Chronobiol Int. 2021. PMID: 33393371
-
The Instigation of the Associations Between Melatonin, Circadian Genes, and Epileptic Spasms in Infant Rats.Front Neurol. 2020 Oct 26;11:497225. doi: 10.3389/fneur.2020.497225. eCollection 2020. Front Neurol. 2020. PMID: 33192961 Free PMC article.
-
USP2 regulates the intracellular localization of PER1 and circadian gene expression.J Biol Rhythms. 2014 Aug;29(4):243-56. doi: 10.1177/0748730414544741. J Biol Rhythms. 2014. PMID: 25238854
-
The Circadian Clock, Nutritional Signals and Reproduction: A Close Relationship.Int J Mol Sci. 2023 Jan 12;24(2):1545. doi: 10.3390/ijms24021545. Int J Mol Sci. 2023. PMID: 36675058 Free PMC article. Review.
-
Molecular Links Between Circadian Rhythm Disruption, Melatonin, and Neurodegenerative Diseases: An Updated Review.Molecules. 2025 Apr 23;30(9):1888. doi: 10.3390/molecules30091888. Molecules. 2025. PMID: 40363695 Free PMC article. Review.
Cited by
-
Why cells need iron: a compendium of iron utilisation.Trends Endocrinol Metab. 2024 Dec;35(12):1026-1049. doi: 10.1016/j.tem.2024.04.015. Epub 2024 May 17. Trends Endocrinol Metab. 2024. PMID: 38760200 Free PMC article. Review.
-
Micronutrients and midnight: the diet-sleep link.Sleep. 2025 Aug 14;48(8):zsaf143. doi: 10.1093/sleep/zsaf143. Sleep. 2025. PMID: 40445882 Free PMC article. No abstract available.
-
Flexible iron: disorder in the ironome brings order to protein structure and function.Front Mol Biosci. 2025 May 30;12:1537164. doi: 10.3389/fmolb.2025.1537164. eCollection 2025. Front Mol Biosci. 2025. PMID: 40520260 Free PMC article.
-
Circadian-Tuned Peptide Drug/Gene Co-Delivery Nanocomplexes to Enhance Glioblastoma Targeting and Transfection.Int J Mol Sci. 2025 Jun 26;26(13):6130. doi: 10.3390/ijms26136130. Int J Mol Sci. 2025. PMID: 40649908 Free PMC article.
References
-
- Nassan M, Videnovic A. Circadian rhythms in neurodegenerative disorders. Nat Rev Neurol. 2022;18:7‐24. - PubMed
-
- Castro‐Zavala A, Alegre‐Zurano L, Cantacorps L, et al. Bmal1‐knockout mice exhibit reduced cocaine‐seeking behaviour and cognitive impairments. Biomed Pharmacother. 2022;153:113333. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- C2023423008/Natural Science Foundation of Hebei Province
- H2019423032/Natural Science Foundation of Hebei Province
- YQ2018007/Basic research program for young teachers of Hebei University of Chinese Medicine
- C2023205049/Hebei Research Center of the Basic Discipline of Cell Biology
- BJK2023095/Science Research Project of Hebei Education Department
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

