The energy-converting hydrogenase Ech2 is important for the growth of the thermophilic acetogen Thermoanaerobacter kivui on ferredoxin-dependent substrates
- PMID: 38385688
- PMCID: PMC10986591
- DOI: 10.1128/spectrum.03380-23
The energy-converting hydrogenase Ech2 is important for the growth of the thermophilic acetogen Thermoanaerobacter kivui on ferredoxin-dependent substrates
Abstract
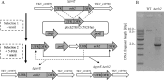
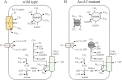
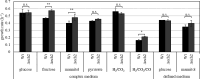
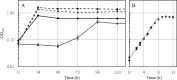
Thermoanaerobacter kivui is the thermophilic acetogenic bacterium with the highest temperature optimum (66°C) and with high growth rates on hydrogen (H2) plus carbon dioxide (CO2). The bioenergetic model suggests that its redox and energy metabolism depends on energy-converting hydrogenases (Ech). Its genome encodes two Echs, Ech1 and Ech2, as sole coupling sites for energy conservation during growth on H2 + CO2. During growth on other substrates, its redox activity, the (proton-gradient-coupled) oxidation of H2 may be essential to provide reduced ferredoxin (Fd) to the cell. While Ech activity has been demonstrated biochemically, the physiological function of both Ech's is unclear. Toward that, we deleted the complete gene cluster encoding Ech2. Surprisingly, the ech2 mutant grew as fast as the wild type on sugar substrates and H2 + CO2. Hence, Ech1 may be the essential enzyme for energy conservation, and either Ech1 or another enzyme may substitute for H2-dependent Fd reduction during growth on sugar substrates, putatively the H2-dependent CO2 reductase (HDCR). Growth on pyruvate and CO, substrates that are oxidized by Fd-dependent enzymes, was significantly impaired, but to a different extent. While ∆ech2 grew well on pyruvate after four transfers, ∆ech2 did not adapt to CO. Cell suspensions of ∆ech2 converted pyruvate to acetate, but no acetate was produced from CO. We analyzed the genome of five T. kivui strains adapted to CO. Strikingly, all strains carried mutations in the hycB3 subunit of HDCR. These mutations are obviously essential for the growth on CO but may inhibit its ability to utilize Fd as substrate.
Importance: Acetogens thrive by converting H2+CO2 to acetate. Under environmental conditions, this allows for only very little energy to be conserved (∆G'<-20 kJ mol-1). CO2 serves as a terminal electron acceptor in the ancient Wood-Ljungdahl pathway (WLP). Since the WLP is ATP neutral, energy conservation during growth on H2 + CO2 is dependent on the redox metabolism. Two types of acetogens can be distinguished, Rnf- and Ech-type. The function of both membrane-bound enzyme complexes is twofold-energy conversion and redox balancing. Ech couples the Fd-dependent reduction of protons to H2 to the formation of a proton gradient in the thermophilic bacterium Thermoanaerobacter kivui. This bacterium may be utilized in gas fermentation at high temperatures, due to very high conversion rates and the availability of genetic tools. The physiological function of an Ech hydrogenase in T. kivui was studied to contribute an understanding of its energy and redox metabolism, a prerequisite for future industrial applications.
Keywords: Thermoanaerobacter kivui; acetogen; carbon monoxide; energy-converting hydrogenase; ferredoxin; pyruvate; thermophilic.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








Similar articles
-
A genome-guided analysis of energy conservation in the thermophilic, cytochrome-free acetogenic bacterium Thermoanaerobacter kivui.BMC Genomics. 2014 Dec 18;15(1):1139. doi: 10.1186/1471-2164-15-1139. BMC Genomics. 2014. PMID: 25523312 Free PMC article.
-
Formate Is Required for Growth of the Thermophilic Acetogenic Bacterium Thermoanaerobacter kivui Lacking Hydrogen-Dependent Carbon Dioxide Reductase (HDCR).Front Microbiol. 2020 Jan 31;11:59. doi: 10.3389/fmicb.2020.00059. eCollection 2020. Front Microbiol. 2020. PMID: 32082286 Free PMC article.
-
A purified energy-converting hydrogenase from Thermoanaerobacter kivui demonstrates coupled H+-translocation and reduction in vitro.J Biol Chem. 2022 Aug;298(8):102216. doi: 10.1016/j.jbc.2022.102216. Epub 2022 Jun 30. J Biol Chem. 2022. PMID: 35779632 Free PMC article.
-
Flavin-Based Electron Bifurcation, Ferredoxin, Flavodoxin, and Anaerobic Respiration With Protons (Ech) or NAD+ (Rnf) as Electron Acceptors: A Historical Review.Front Microbiol. 2018 Mar 14;9:401. doi: 10.3389/fmicb.2018.00401. eCollection 2018. Front Microbiol. 2018. PMID: 29593673 Free PMC article. Review.
-
Energy conservation via electron bifurcating ferredoxin reduction and proton/Na(+) translocating ferredoxin oxidation.Biochim Biophys Acta. 2013 Feb;1827(2):94-113. doi: 10.1016/j.bbabio.2012.07.002. Epub 2012 Jul 16. Biochim Biophys Acta. 2013. PMID: 22800682 Review.
Cited by
-
Isolation and characterization of novel acetogenic Moorella strains for employment as potential thermophilic biocatalysts.FEMS Microbiol Ecol. 2024 Aug 13;100(9):fiae109. doi: 10.1093/femsec/fiae109. FEMS Microbiol Ecol. 2024. PMID: 39118367 Free PMC article.
-
Hi-TARGET: a fast, efficient and versatile CRISPR type I-B genome editing tool for the thermophilic acetogen Thermoanaerobacter kivui.Biotechnol Biofuels Bioprod. 2025 Apr 30;18(1):49. doi: 10.1186/s13068-025-02647-0. Biotechnol Biofuels Bioprod. 2025. PMID: 40307869 Free PMC article.
-
Redirecting electron flow in Acetobacterium woodii enables growth on CO and improves growth on formate.Nat Commun. 2024 Jun 26;15(1):5424. doi: 10.1038/s41467-024-49680-5. Nat Commun. 2024. PMID: 38926344 Free PMC article.
-
Isolation and characterization of novel acetogenic strains of the genera Terrisporobacter and Acetoanaerobium.Front Microbiol. 2024 Jul 3;15:1426882. doi: 10.3389/fmicb.2024.1426882. eCollection 2024. Front Microbiol. 2024. PMID: 39021630 Free PMC article.
-
A megatransposon drives the adaptation of Thermoanaerobacter kivui to carbon monoxide.Nat Commun. 2025 May 6;16(1):4217. doi: 10.1038/s41467-025-59103-8. Nat Commun. 2025. PMID: 40328730 Free PMC article.
References
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

