Discovery of Two Novel Immunoepitopes and Development of a Peptide-based Sarcoidosis Immunoassay
- PMID: 38385694
- PMCID: PMC11506913
- DOI: 10.1164/rccm.202306-1054OC
Discovery of Two Novel Immunoepitopes and Development of a Peptide-based Sarcoidosis Immunoassay
Abstract
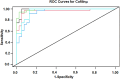
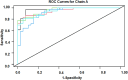
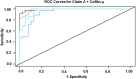
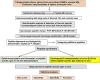
Rationale: Sarcoidosis is a systemic granulomatous disorder associated with hypergammaglobulinemia and the presence of autoantibodies. The specific antigens initiating granulomatous inflammation in sarcoidosis are unknown, and there is no specific test available to diagnose sarcoidosis. To discover novel sarcoidosis antigens, we developed a high-throughput T7 phage display library derived from the sarcoidosis cDNA and identified numerous clones differentiating sarcoidosis from other respiratory diseases. After clone sequencing and a homology search, we identified two epitopes (cofilin μ and chain A) that specifically bind to serum IgGs of patients with sarcoidosis. Objectives: To develop and validate an epitope-specific IgG-based immunoassay specific for sarcoidosis. Methods: We chemically synthesized both immunoepitopes (cofilin μ and chain A) and generated rabbit polyclonal antibodies against both neoantigens. After extensive standardization, we developed a direct peptide ELISA and measured epitope-specific IgG in the sera of 386 subjects, including healthy control subjects (n = 100), three sarcoidosis cohorts (n = 186), pulmonary tuberculosis (n = 70), and lung cancer (n = 30). Measurements and Main Results: To develop a model to classify sarcoidosis distinctly from other groups, data were analyzed using fivefold cross-validation when adjusting for confounders. The cofilin μ IgG model yielded a mean sensitivity, specificity, and positive and negative predictive value of 0.97, 0.9, 0.9, and 0.96, respectively. Those same measures for chain A IgG antibody were 0.9, 0.83, 0.84, and 0.9, respectively. Combining both biomarkers improved the area under the curve, sensitivity, specificity, and positive and negative predictive value. Conclusions: These results provide a novel immunoassay for sarcoidosis. The discovery of two neoantigens facilitates the development of biospecific drug discovery and the sarcoidosis-specific model.
Keywords: T7 phage display; antigen; chain A; cofilin; sarcoidosis.
Figures


Comment in
-
A Promising Biomarker for Pulmonary Sarcoidosis That Must Cross the Finish Line.Am J Respir Crit Care Med. 2024 Oct 1;210(7):863-864. doi: 10.1164/rccm.202404-0750ED. Am J Respir Crit Care Med. 2024. PMID: 38748223 Free PMC article. No abstract available.
References
-
- Häggmark A, Hamsten C, Wiklundh E, Lindskog C, Mattsson C, Andersson E, et al. Proteomic profiling reveals autoimmune targets in sarcoidosis. Am J Respir Crit Care Med . 2015;191:574–583. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

