Effect of smoking cessation interventions on abstinence and tuberculosis treatment outcomes among newly diagnosed patients: a randomized controlled trial
- PMID: 38385711
- PMCID: PMC10986535
- DOI: 10.1128/spectrum.03878-23
Effect of smoking cessation interventions on abstinence and tuberculosis treatment outcomes among newly diagnosed patients: a randomized controlled trial
Abstract
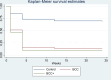
The study evaluates the effectiveness of smoking cessation interventions [Behavioral Change Communication (BCC) and Behavioral Change Communication plus bupropion (BCC+)] compared to conventional Directly Observed Therapy Short Course (DOT) treatment in improving pulmonary tuberculosis treatment outcomes and abstinence among newly diagnosed pulmonary tuberculosis (PTB) patients, highlighting the scarcity of robust experimental studies. The current randomized controlled trial, conducted at Ojha Institute of Chest Diseases between October 2017 and June 2019, randomized 292 patients who were current smokers with newly diagnosed pulmonary tuberculosis into three arms: control (n = 97), BCC (n = 97), and BCC+ (n = 98) arms. The outcomes of the interventions were compared in terms of favorable treatment outcomes and abstinence achieved at the end of 6 months. Baseline characteristics were compared between groups. Cox regression quantified the effect size of interventions for both outcome variables and reported as (crude and adjusted) hazard ratios with 95% confidence intervals (CI). No statistically significant difference was observed in baseline characteristics in each arm. Both BCC+ and BCC showed a statistically significant effect in achieving favorable PTB outcomes at 6 months (aHR 2.37, 95% CI 1.52-3.70 and aHR 2.34, 95% CI 1.51-3.60), as well as for abstinence from smoking at 6 months (BCC+: aHR 4.03, 95% CI 2.18-7.44 and BCC: aHR 3.87, 95% CI 2.12-7.05) compared to the control arm. Both BCC and BCC+ aided by pharmacologic agents such as bupropion when incorporated with conventional DOTs were found to be significantly effective in attaining favorable tuberculosis treatment outcomes as well as in attaining smoking abstinence at the end of the 6-month treatment.
This study shows that adding smoking cessation programs (with or without extra drugs like bupropion) to standard Directly Observed Treatment Short Course (DOTs) treatment for people who have recently been diagnosed with pulmonary tuberculosis has a great positive impact on how well the overall antituberculosis treatment works. Our trial shows very promising results for such a combined therapy (DOTs and smoking cessation) in a country where the burden of both tuberculosis and smoking is very high.
Keywords: behavioral change communication; smoking cessation interventions; smoking treatment outcomes; tuberculosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Interventions for preventing weight gain after smoking cessation.Cochrane Database Syst Rev. 2012 Jan 18;1:CD006219. doi: 10.1002/14651858.CD006219.pub3. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2021 Oct 6;10:CD006219. doi: 10.1002/14651858.CD006219.pub4. PMID: 22258966 Updated.
-
Smoking cessation medicines and e-cigarettes: a systematic review, network meta-analysis and cost-effectiveness analysis.Health Technol Assess. 2021 Oct;25(59):1-224. doi: 10.3310/hta25590. Health Technol Assess. 2021. PMID: 34668482
-
Interventions for preventing weight gain after smoking cessation.Cochrane Database Syst Rev. 2009 Jan 21;(1):CD006219. doi: 10.1002/14651858.CD006219.pub2. Cochrane Database Syst Rev. 2009. Update in: Cochrane Database Syst Rev. 2012 Jan 18;1:CD006219. doi: 10.1002/14651858.CD006219.pub3. PMID: 19160269 Updated.
-
Electronic cigarettes for smoking cessation and reduction.Cochrane Database Syst Rev. 2014;(12):CD010216. doi: 10.1002/14651858.CD010216.pub2. Epub 2014 Dec 17. Cochrane Database Syst Rev. 2014. PMID: 25515689
-
Interventions for preventing weight gain after smoking cessation.Cochrane Database Syst Rev. 2021 Oct 6;10(10):CD006219. doi: 10.1002/14651858.CD006219.pub4. Cochrane Database Syst Rev. 2021. PMID: 34611902 Free PMC article.
Cited by
-
Epidemiology and Molecular Drug-Resistance Patterns of Tuberculosis in Non-Elderly Patients in Luoyang, China, 2019-2023.Infect Drug Resist. 2025 Jun 24;18:3087-3101. doi: 10.2147/IDR.S524300. eCollection 2025. Infect Drug Resist. 2025. PMID: 40590043 Free PMC article.
-
Unveiling the Long-Term Lung Consequences of Smoking and Tobacco Consumption: A Narrative Review.Cureus. 2024 Aug 8;16(8):e66415. doi: 10.7759/cureus.66415. eCollection 2024 Aug. Cureus. 2024. PMID: 39246889 Free PMC article. Review.
References
-
- Sharma SK, Mohan A, Singh AD, Mishra H, Jhanjee S, Pandey RM, Singh BK, Sharma R, Pallipamu PB, Pai M, Dheda K. 2018. Impact of nicotine replacement therapy as an adjunct to anti-tuberculosis treatment and behaviour change counselling in newly diagnosed pulmonary tuberculosis patients: an open-label, randomised controlled trial. Sci Rep 8:8828. doi:10.1038/s41598-018-26990-5 - DOI - PMC - PubMed
-
- Siddiqui UA, O’Toole M, Kabir Z, Qureshi S, Gibbons N, Kane M, Keane J. 2010. Smoking prolongs the infectivity of patients with tuberculosis. Ir Med J 103:278–280. - PubMed
-
- Altet N, Latorre I, Jiménez-Fuentes MÁ, Maldonado J, Molina I, González-Díaz Y, Milà C, García-García E, Muriel B, Villar-Hernández R, Laabei M, Gómez A-C, Godoy P, de Souza-Galvão ML, Solano S, Jiménez-Ruiz CA, Domínguez J, PII Smoking SEPAR Working Group . 2017. Assessment of the influence of direct tobacco smoke on infection and active TB management. PLoS One 12:e0182998. doi:10.1371/journal.pone.0182998 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical