High-dose atorvastatin therapy progressively decreases skeletal muscle mitochondrial respiratory capacity in humans
- PMID: 38385748
- PMCID: PMC10967389
- DOI: 10.1172/jci.insight.174125
High-dose atorvastatin therapy progressively decreases skeletal muscle mitochondrial respiratory capacity in humans
Abstract
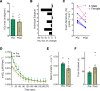
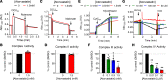
BACKGROUNDWhile the benefits of statin therapy on atherosclerotic cardiovascular disease are clear, patients often experience mild to moderate skeletal myopathic symptoms, the mechanism for which is unknown. This study investigated the potential effect of high-dose atorvastatin therapy on skeletal muscle mitochondrial function and whole-body aerobic capacity in humans.METHODSEight overweight (BMI, 31.9 ± 2.0) but otherwise healthy sedentary adults (4 females, 4 males) were studied before (day 0) and 14, 28, and 56 days after initiating atorvastatin (80 mg/d) therapy.RESULTSMaximal ADP-stimulated respiration, measured in permeabilized fiber bundles from muscle biopsies taken at each time point, declined gradually over the course of atorvastatin treatment, resulting in > 30% loss of skeletal muscle mitochondrial oxidative phosphorylation capacity by day 56. Indices of in vivo muscle oxidative capacity (via near-infrared spectroscopy) decreased by 23% to 45%. In whole muscle homogenates from day 0 biopsies, atorvastatin inhibited complex III activity at midmicromolar concentrations, whereas complex IV activity was inhibited at low nanomolar concentrations.CONCLUSIONThese findings demonstrate that high-dose atorvastatin treatment elicits a striking progressive decline in skeletal muscle mitochondrial respiratory capacity, highlighting the need for longer-term dose-response studies in different patient populations to thoroughly define the effect of statin therapy on skeletal muscle health.FUNDINGNIH R01 AR071263.
Keywords: Bioenergetics; Mitochondria; Muscle biology; Skeletal muscle.
Conflict of interest statement
Figures

References
-
- Grundy SM, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139(25):e1082–e1143. doi: 10.1161/CIR.0000000000000625. - DOI - PMC - PubMed
-
- European Association for Cardiovascular P. et al. ESC/EAS Guidelines for the management of dyslipidaemias: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS) Eur Heart J. 2011;32(14):1769–1818. doi: 10.1093/eurheartj/ehr158. - DOI - PubMed

