Lactobacillus acidophilus KBL409 Ameliorates Atopic Dermatitis in a Mouse Model
- PMID: 38386273
- PMCID: PMC11021314
- DOI: 10.1007/s12275-024-00104-5
Lactobacillus acidophilus KBL409 Ameliorates Atopic Dermatitis in a Mouse Model
Abstract
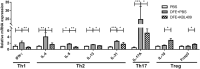
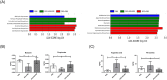
Atopic dermatitis (AD) is a chronic inflammatory skin disease with repeated exacerbations of eczema and pruritus. Probiotics can prevent or treat AD appropriately via modulation of immune responses and gut microbiota. In this study, we evaluated effects of Lactobacillus acidophilus (L. acidophilus) KBL409 using a house dust mite (Dermatophagoides farinae)-induced in vivo AD model. Oral administration of L. acidophilus KBL409 significantly reduced dermatitis scores and decreased infiltration of immune cells in skin tissues. L. acidophilus KBL409 reduced in serum immunoglobulin E and mRNA levels of T helper (Th)1 (Interferon-γ), Th2 (Interleukin [IL]-4, IL-5, IL-13, and IL-31), and Th17 (IL-17A) cytokines in skin tissues. The anti-inflammatory cytokine IL-10 was increased and Foxp3 expression was up-regulated in AD-induced mice with L. acidophilus KBL409. Furthermore, L. acidophilus KBL409 significantly modulated gut microbiota and concentrations of short-chain fatty acids and amino acids, which could explain its effects on AD. Our results suggest that L. acidophilus KBL409 is the potential probiotic for AD treatment by modulating of immune responses and gut microbiota of host.
Keywords: Lactobacillus acidophilus; Atopic dermatitis; Gut-skin axis; Immune response; Microbiota; Probiotic.
© 2024. The Author(s).
Conflict of interest statement
G.K. is the founder of KoBioLabs, Inc, and S.P. is an employee of KoBioLabs, Inc. The other authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Fania L, Moretta G, Antonelli F, Scala E, Abeni D, Albanesi C, Madonna S. Multiple roles for cytokines in atopic dermatitis: From pathogenic mediators to endotype-specific biomarkers to therapeutic targets. International Journal of Molecular Sciences. 2022;23:2684. doi: 10.3390/ijms23052684. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

