A Cancer Nanovaccine for Co-Delivery of Peptide Neoantigens and Optimized Combinations of STING and TLR4 Agonists
- PMID: 38386282
- PMCID: PMC10919087
- DOI: 10.1021/acsnano.3c04471
A Cancer Nanovaccine for Co-Delivery of Peptide Neoantigens and Optimized Combinations of STING and TLR4 Agonists
Abstract
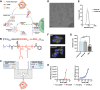
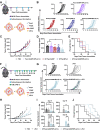
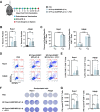
Immune checkpoint blockade (ICB) has revolutionized cancer treatment and led to complete and durable responses, but only for a minority of patients. Resistance to ICB can largely be attributed to insufficient number and/or function of antitumor CD8+ T cells in the tumor microenvironment. Neoantigen targeted cancer vaccines can activate and expand the antitumor T cell repertoire, but historically, clinical responses have been poor because immunity against peptide antigens is typically weak, resulting in insufficient activation of CD8+ cytotoxic T cells. Herein, we describe a nanoparticle vaccine platform that can overcome these barriers in several ways. First, the vaccine can be reproducibly formulated using a scalable confined impingement jet mixing method to coload a variety of physicochemically diverse peptide antigens and multiple vaccine adjuvants into pH-responsive, vesicular nanoparticles that are monodisperse and less than 100 nm in diameter. Using this approach, we encapsulated synergistically acting adjuvants, cGAMP and monophosphoryl lipid A (MPLA), into the nanocarrier to induce a robust and tailored innate immune response that increased peptide antigen immunogenicity. We found that incorporating both adjuvants into the nanovaccine synergistically enhanced expression of dendritic cell costimulatory markers, pro-inflammatory cytokine secretion, and peptide antigen cross-presentation. Additionally, the nanoparticle delivery increased lymph node accumulation and uptake of peptide antigen by dendritic cells in the draining lymph node. Consequently, nanoparticle codelivery of peptide antigen, cGAMP, and MPLA enhanced the antigen-specific CD8+ T cell response and delayed tumor growth in several mouse models. Finally, the nanoparticle platform improved the efficacy of ICB immunotherapy in a murine colon carcinoma model. This work establishes a versatile nanoparticle vaccine platform for codelivery of peptide neoantigens and synergistic adjuvants to enhance responses to cancer vaccines.
Keywords: adjuvant synergy; cancer vaccine; endosomal escape; immune checkpoint blockade; immunotherapy; nanoparticle.
Conflict of interest statement
The authors declare the following competing financial interest(s): J.T.W. is an inventor on U.S. Patent 10,696,985 Reversibly Crosslinked Endosomolytic Polymer Vesicles for Cytosolic Drug Delivery and on U.S. Patent Application PCT/US2019/058945 Graft Copolymers, Methods of Forming Graft Copolymers, and Methods of Use Thereof, both of which describe drug delivery technologies that have been used for STING agonist delivery.
Figures


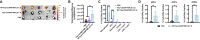

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

