Inverse regulation of SOS1 and HKT1 protein localization and stability by SOS3/CBL4 in Arabidopsis thaliana
- PMID: 38386704
- PMCID: PMC10907282
- DOI: 10.1073/pnas.2320657121
Inverse regulation of SOS1 and HKT1 protein localization and stability by SOS3/CBL4 in Arabidopsis thaliana
Abstract
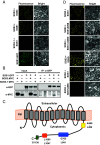
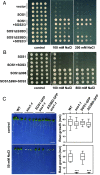
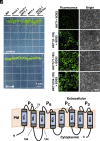
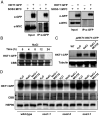
To control net sodium (Na+) uptake, Arabidopsis plants utilize the plasma membrane (PM) Na+/H+ antiporter SOS1 to achieve Na+ efflux at the root and Na+ loading into the xylem, and the channel-like HKT1;1 protein that mediates the reverse flux of Na+ unloading off the xylem. Together, these opposing transport systems govern the partition of Na+ within the plant yet they must be finely co-regulated to prevent a futile cycle of xylem loading and unloading. Here, we show that the Arabidopsis SOS3 protein acts as the molecular switch governing these Na+ fluxes by favoring the recruitment of SOS1 to the PM and its subsequent activation by the SOS2/SOS3 kinase complex under salt stress, while commanding HKT1;1 protein degradation upon acute sodic stress. SOS3 achieves this role by direct and SOS2-independent binding to previously unrecognized functional domains of SOS1 and HKT1;1. These results indicate that roots first retain moderate amounts of salts to facilitate osmoregulation, yet when sodicity exceeds a set point, SOS3-dependent HKT1;1 degradation switches the balance toward Na+ export out of the root. Thus, SOS3 functionally links and co-regulates the two major Na+ transport systems operating in vascular plants controlling plant tolerance to salinity.
Keywords: Arabidopsis; HKT1; SOS pathway; salinity; sodium transport.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures



References
-
- Cheeseman J. M., The evolution of halophytes, glycophytes and crops, and its implications for food security under saline conditions. New Phytol. 206, 557–570 (2015). - PubMed
-
- Ismail A. M., Horie T., Genomics, physiology, and molecular breeding approaches for improving salt tolerance. Annu. Rev. Plant Biol. 68, 405–434 (2017). - PubMed
-
- Ji H., et al. , The Salt Overly Sensitive (SOS) pathway: Established and emerging roles. Mol. Plant 6, 275–286 (2013). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

