Tissue-Resident Alveolar Macrophages Reduce Ozone-induced Inflammation via MerTK-mediated Efferocytosis
- PMID: 38386777
- PMCID: PMC11160417
- DOI: 10.1165/rcmb.2023-0390OC
Tissue-Resident Alveolar Macrophages Reduce Ozone-induced Inflammation via MerTK-mediated Efferocytosis
Abstract
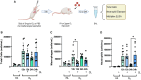
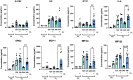
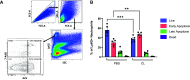
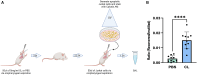
Lung inflammation, caused by acute exposure to ozone (O3), one of the six criteria air pollutants, is a significant source of morbidity in susceptible individuals. Alveolar macrophages (AMØs) are the most abundant immune cells in the normal lung, and their number increases after O3 exposure. However, the role of AMØs in promoting or limiting O3-induced lung inflammation has not been clearly defined. In this study, we used a mouse model of acute O3 exposure, lineage tracing, genetic knockouts, and data from O3-exposed human volunteers to define the role and ontogeny of AMØs during acute O3 exposure. Lineage-tracing experiments showed that 12, 24, and 72 hours after exposure to O3 (2 ppm) for 3 hours, all AMØs were of tissue-resident origin. Similarly, in humans exposed to filtered air and O3 (200 ppb) for 135 minutes, we did not observe at ∼21 hours postexposure an increase in monocyte-derived AMØs by flow cytometry. Highlighting a role for tissue-resident AMØs, we demonstrate that depletion of tissue-resident AMØs with clodronate-loaded liposomes led to persistence of neutrophils in the alveolar space after O3 exposure, suggesting that impaired neutrophil clearance (i.e., efferocytosis) leads to prolonged lung inflammation. Moreover, depletion of tissue-resident AMØs demonstrated reduced clearance of intratracheally instilled apoptotic Jurkat cells, consistent with reduced efferocytosis. Genetic ablation of MerTK (MER proto-oncogene, tyrosine kinase), a key receptor involved in efferocytosis, also resulted in impaired clearance of apoptotic neutrophils after O3 exposure. Overall, these findings underscore the pivotal role of tissue-resident AMØs in resolving O3-induced inflammation via MerTK-mediated efferocytosis.
Keywords: MerTK; efferocytosis; macrophages; ozone.
Figures




Update of
-
Tissue-resident alveolar macrophages reduce O3-induced inflammation via MerTK mediated efferocytosis.bioRxiv [Preprint]. 2023 Nov 6:2023.11.06.565865. doi: 10.1101/2023.11.06.565865. bioRxiv. 2023. Update in: Am J Respir Cell Mol Biol. 2024 Jun;70(6):493-506. doi: 10.1165/rcmb.2023-0390OC. PMID: 37986982 Free PMC article. Updated. Preprint.
Comment in
-
Cell-Corpse Clearance after Lung Damage: The Essential Role of MerTK-mediated Alveolar Macrophage Efferocytosis.Am J Respir Cell Mol Biol. 2024 Jun;70(6):433-434. doi: 10.1165/rcmb.2024-0108ED. Am J Respir Cell Mol Biol. 2024. PMID: 38502903 Free PMC article. No abstract available.
References
-
- Fuller R, Landrigan PJ, Balakrishnan K, Bathan G, Bose-O’Reilly S, Brauer M, et al. Pollution and health: a progress update. Lancet Planet Health . 2022;6:e535–e547. - PubMed
-
- Liao H, Chen WT, Seinfeld JH. Role of climate change in global predictions of future tropospheric ozone and aerosols. J Geophys Res Atmos . 2006;111:D12304.
-
- Samet JM, Zeger SL, Dominici F, Curriero F, Coursac I, Dockery DW, et al. The National Morbidity, Mortality, and Air Pollution Study. Part II: morbidity and mortality from air pollution in the United States. Res Rep Health Eff Inst . 2000;94:5–70. - PubMed
MeSH terms
Substances
Grants and funding
- R01 ES034350/ES/NIEHS NIH HHS/United States
- P01HL154998/NH/NIH HHS/United States
- Z01 ES102005/ES/NIEHS NIH HHS/United States
- S10 OD011996/OD/NIH HHS/United States
- R01 HL153312/HL/NHLBI NIH HHS/United States
- P01 HL154998/HL/NHLBI NIH HHS/United States
- R01 ES028829/ES/NIEHS NIH HHS/United States
- T32ES021432/ES/NIEHS NIH HHS/United States
- R21 AG075423/AG/NIA NIH HHS/United States
- P01AG049665/NH/NIH HHS/United States
- R01ES027574/NH/NIH HHS/United States
- U19AI135964/NH/NIH HHS/United States
- R01 ES027574/ES/NIEHS NIH HHS/United States
- P01 AG049665/AG/NIA NIH HHS/United States
- U19 AI135964/AI/NIAID NIH HHS/United States
- T32 ES021432/ES/NIEHS NIH HHS/United States
- Z01 ES102005/ImNIH/Intramural NIH HHS/United States
- R01ES034350/NH/NIH HHS/United States
- R01 HL158139/HL/NHLBI NIH HHS/United States
- P30 CA060553/CA/NCI NIH HHS/United States
- R01HL158139/NH/NIH HHS/United States
- R21AG075423/NH/NIH HHS/United States
- R01HL153312/NH/NIH HHS/United States
- R01ES028829/NH/NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

