Sacituzumab govitecan plus platinum-based chemotherapy mediates significant antitumor effects in triple-negative breast, urinary bladder, and small-cell lung carcinomas
- PMID: 38386805
- PMCID: PMC10883684
- DOI: 10.18632/oncotarget.28559
Sacituzumab govitecan plus platinum-based chemotherapy mediates significant antitumor effects in triple-negative breast, urinary bladder, and small-cell lung carcinomas
Abstract
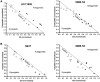
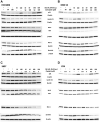
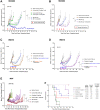
Sacituzumab govitecan (SG) is an antibody-drug conjugate composed of an anti-Trop-2-directed antibody conjugated with the topoisomerase I inhibitory drug, SN-38, via a proprietary hydrolysable linker. SG has received United States Food and Drug Administration (FDA) approval to treat metastatic triple-negative breast cancer (TNBC), unresectable locally advanced or metastatic hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative breast cancer, and accelerated approval for metastatic urothelial cancer. We investigated the utility of combining SG with platinum-based chemotherapeutics in TNBC, urinary bladder carcinoma (UBC), and small-cell lung carcinoma (SCLC). SG plus carboplatin or cisplatin produced additive growth-inhibitory effects in vitro that trended towards synergy. Immunoblot analysis of cell lysates suggests perturbation of the cell-cycle and a shift towards pro-apoptotic signaling evidenced by an increased Bax to Bcl-2 ratio and down-regulation of two anti-apoptotic proteins, Mcl-1 and survivin. Significant antitumor effects were observed with SG plus carboplatin in mice bearing TNBC or SCLC tumors compared to all controls (P < 0.0062 and P < 0.0017, respectively) and with SG plus cisplatin in UBC and SCLC tumor-bearing animals (P < 0.0362 and P < 0.0001, respectively). These combinations were well tolerated by the animals. Combining SG with platinum-based chemotherapeutics demonstrates the benefit in these indications and warrants further clinical investigation.
Keywords: SN-38; Trop-2; carboplatin; cisplatin; sacituzumab govitecan.
Conflict of interest statement
All authors were employed by Immunomedics, Inc. at the time this work was performed. Thomas M. Cardillo is currently a contract employee of Gilead Sciences, Inc., David M. Goldenberg holds royalty rights, as well as other payment rights, and Serengulam V. Govindan owns stock in Gilead Sciences, Inc.
Figures
References
-
- Cardillo TM, Govindan SV, Sharkey RM, Trisal P, Goldenberg DM. Humanized anti-Trop-2 IgG-SN-38 conjugate for effective treatment of diverse epithelial cancers: preclinical studies in human cancer xenograft models and monkeys. Clin Cancer Res. 2011; 17:3157–69. 10.1158/1078-0432.CCR-10-2939. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

